| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2009-07-23 18:26:15 UTC |
|---|
| Update Date | 2016-11-09 01:08:46 UTC |
|---|
| Accession Number | CHEM002433 |
|---|
| Identification |
|---|
| Common Name | Alcuronium |
|---|
| Class | Small Molecule |
|---|
| Description | Alcuronium is a type of curare, a plant toxin known for its use as paralyzing arrow poison by South American indigenous people. It can be extracted from a variety of plants, including Strychnos toxifera and Chondrodendron tomentosum. Curares are active only by an injection. They are harmless if taken orally because curare compounds are too large and too highly charged to pass through the lining of the digestive tract to be absorbed into the blood. (1) |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- T3DB toxins
|
|---|
| Contaminant Type | - Amine
- Bromide Compound
- Natural Compound
- Organic Compound
- Plant Toxin
|
|---|
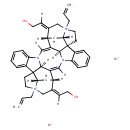
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C44H50Br2N4O2 |
|---|
| Average Molecular Mass | 826.701 g/mol |
|---|
| Monoisotopic Mass | 824.230 g/mol |
|---|
| CAS Registry Number | 23214-96-2 |
|---|
| IUPAC Name | (1S,9Z,11S,13S,25Z,27S,28E,33S,35S,37E,38S)-28,37-bis(2-hydroxyethylidene)-14,30-bis(prop-2-en-1-yl)-8,14,24,30-tetraazaundecacyclo[25.5.2.2¹¹,¹⁴.1¹,⁸.1¹⁰,¹⁷.0²,⁷.0¹³,¹⁷.0¹⁸,²³.0³⁰,³³.0²⁴,³⁵.0²⁶,³⁸]octatriaconta-2,4,6,9,18,20,22,25-octaene-14,30-diium dibromide |
|---|
| Traditional Name | (1S,9Z,11S,13S,25Z,27S,28E,33S,35S,37E,38S)-28,37-bis(2-hydroxyethylidene)-14,30-bis(prop-2-en-1-yl)-8,14,24,30-tetraazaundecacyclo[25.5.2.2¹¹,¹⁴.1¹,⁸.1¹⁰,¹⁷.0²,⁷.0¹³,¹⁷.0¹⁸,²³.0³⁰,³³.0²⁴,³⁵.0²⁶,³⁸]octatriaconta-2,4,6,9,18,20,22,25-octaene-14,30-diium dibromide |
|---|
| SMILES | [Br-].[Br-].[H]\C(CO)=C1/C[N+]2(CC=C)CCC34C5=CC=CC=C5N5\C([H])=C6/[C@]7([H])N(\C([H])=C(/[C@@]35[H])[C@@]1([H])C[C@]24[H])C1=CC=CC=C1[C@@]71CC[N+]2(CC=C)C\C(=C(/[H])CO)[C@]6([H])C[C@@]12[H] |
|---|
| InChI Identifier | InChI=1S/C44H50N4O2.2BrH/c1-3-17-47-19-15-43-35-9-5-7-11-37(35)45-26-34-32-24-40-44(16-20-48(40,18-4-2)28-30(32)14-22-50)36-10-6-8-12-38(36)46(42(34)44)25-33(41(43)45)31(23-39(43)47)29(27-47)13-21-49;;/h3-14,25-26,31-32,39-42,49-50H,1-2,15-24,27-28H2;2*1H/q+2;;/p-2/b29-13-,30-14-,33-25-,34-26-;;/t31-,32-,39-,40-,41-,42-,43+,44?,47?,48?;;/m0../s1 |
|---|
| InChI Key | BXTUHNWXQLWICJ-FRBQZQGESA-L |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as strychnos alkaloids. These are alkaloids having a core structure based on the strychnan, stemmadenine (seco-curan), or the akuammicine (curan) skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Strychnos alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Strychnos alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Strychnan skeleton
- Akuammicine-skeleton
- Stemmadenine-skeleton
- Curan skeleton
- Carbazole
- Indolizidine
- Indole or derivatives
- Tertiary aliphatic/aromatic amine
- Aralkylamine
- Benzenoid
- N-alkylpyrrolidine
- Piperidine
- Tetraalkylammonium salt
- Quaternary ammonium salt
- Pyrrolidine
- Tertiary amine
- Allylamine
- Azacycle
- Organoheterocyclic compound
- Enamine
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organic bromide salt
- Organic salt
- Organic zwitterion
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Amine
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Penicillins | Not Available | Not Available |
|
|---|
| Applications | |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0000000960-a765e2854a5dc0c3c867 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ar0-2200002910-dfdcbb90dc1ed8d6dfc2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9103006500-b07e16c1b4a526e281af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000000390-604df025185c01223197 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dl-0000000940-e944f542ba924455b9b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014r-1302110900-f665118bbce154c62962 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Injection (sting/bite) (1) |
|---|
| Mechanism of Toxicity | Curare is a non-depolarizing muscle relaxant that acts as a competitive antagonist at the nicotinic acetylcholine receptors. (1) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Alcuronium is a type of curare, a plant toxin known for its use as paralyzing arrow poison by South American indigenous people. It can be extracted from a variety of plants, including Strychnos toxifera and Chondrodendron tomentosum. (1) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Curare is a muscle relaxant that can lead to death from asphyxiation, as the respiratory muscles are unable to contract. (1) |
|---|
| Symptoms | Curare is a muscle relaxant and thus causes paraylsis. (1) |
|---|
| Treatment | The antidote for curare poisoning is an acetylcholinesterase inhibitor (anti-cholinesterase), such as physostigmine or neostigmine. (1) |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Alcuronium chloride |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 44427887 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|