| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2009-07-21 20:26:40 UTC |
|---|
| Update Date | 2016-11-09 01:08:42 UTC |
|---|
| Accession Number | CHEM002179 |
|---|
| Identification |
|---|
| Common Name | Trimethadione |
|---|
| Class | Small Molecule |
|---|
| Description | Trimethadione is only found in individuals that have used or taken this drug. It is an anticonvulsant effective in absence seizures, but generally reserved for refractory cases because of its toxicity. (From AMA Drug Evaluations Annual, 1994, p378) Trimethadione reduces T-type calcium currents in thalamic neurons, including thalamic relay neurons. It does so via the inhibition of voltage dependent T-type calcium channels. This raises the threshold for repetitive activity in the thalamus, and inhibits corticothalamic transmission. Thus, the abnormal thalamocortical rhythmicity, which is thought to underlie the 3-Hz spike-and-wave discharge seen on electroencephalogram(EEG) with absence seizures, is dampened. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amide
- Amine
- Anticonvulsant
- Drug
- Ester
- Ether
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
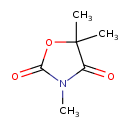
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Tridione | Kegg | | Trimetadione | HMDB | | Trimethadion | HMDB | | Trimethdione | HMDB | | Abbott brand OF trimethadione | HMDB | | Trimetin | HMDB | | Troxidone | HMDB |
|
|---|
| Chemical Formula | C6H9NO3 |
|---|
| Average Molecular Mass | 143.141 g/mol |
|---|
| Monoisotopic Mass | 143.058 g/mol |
|---|
| CAS Registry Number | 127-48-0 |
|---|
| IUPAC Name | trimethyl-1,3-oxazolidine-2,4-dione |
|---|
| Traditional Name | trimethadione |
|---|
| SMILES | CN1C(=O)OC(C)(C)C1=O |
|---|
| InChI Identifier | InChI=1S/C6H9NO3/c1-6(2)4(8)7(3)5(9)10-6/h1-3H3 |
|---|
| InChI Key | IRYJRGCIQBGHIV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oxazolidinediones. Oxazolidinediones are compounds containing an oxazolidine ring which bears two ketones. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azolidines |
|---|
| Sub Class | Oxazolidines |
|---|
| Direct Parent | Oxazolidinediones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oxazolidinedione
- Dicarboximide
- Carbamic acid ester
- Carbonic acid derivative
- Oxacycle
- Azacycle
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Carbonyl group
- Organic oxide
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 46°C | | Boiling Point | Not Available | | Solubility | 5E+004 mg/L |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4l-8900000000-43db0866b0f6797cbff6 | Spectrum | | GC-MS | GC-MS Spectrum - CI-B (Non-derivatized) | splash10-0006-1900000000-5560efcfc7260e1dcfef | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4l-8900000000-43db0866b0f6797cbff6 | Spectrum | | GC-MS | GC-MS Spectrum - CI-B (Non-derivatized) | splash10-0006-1900000000-5560efcfc7260e1dcfef | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052f-9100000000-88bba4b2f392389bb2d0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-1900000000-cadbfe428b3321b1cc08 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0900000000-5ed5d9dd884dba589024 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006x-9000000000-d3d40f4617407850f4a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-2900000000-0d39e71814a5ed15503f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-6900000000-690c1be64c11bb697283 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-79613f1372991c39908a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-1900000000-bf6efd173e2ec81ec24b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0005-9100000000-7199f0f2792c2c7abbf3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-e010af24e65b7370041d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-4900000000-3e2daa1ffb23c9cf8e6c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9100000000-54c03842913d8ee3d521 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9000000000-0173eb4fd0e871a137e0 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0006-9200000000-46082deb9c636fb8f625 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Dione anticonvulsants reduce T-type calcium currents in thalamic neurons, including thalamic relay neurons. It does so via the inhibition of voltage dependent T-type calcium channels. This raises the threshold for repetitive activity in the thalamus, and inhibits corticothalamic transmission. Thus, the abnormal thalamocortical rhythmicity, which is thought to underlie the 3-Hz spike-and-wave discharge seen on electroencephalogram(EEG) with absence seizures, is dampened. |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Used in the control of absence (petit mal) seizures that are refractory to treatment with other medications. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | May cause a potentially dangerous rash that may develop into Stevens Johnson syndrome, an extremely rare but potentially fatal skin disease. |
|---|
| Symptoms | Symptoms of overdose include clumsiness or unsteadiness, coma, dizziness (severe), drowsiness (severe), nausea (severe), and problems with vision. |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00347 |
|---|
| HMDB ID | HMDB0014491 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Trimethadione |
|---|
| Chemspider ID | 5374 |
|---|
| ChEBI ID | 131804 |
|---|
| PubChem Compound ID | 5576 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|