| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2009-06-17 23:53:03 UTC |
|---|
| Update Date | 2016-11-09 01:08:22 UTC |
|---|
| Accession Number | CHEM000833 |
|---|
| Identification |
|---|
| Common Name | Fosamine |
|---|
| Class | Small Molecule |
|---|
| Description | Fosamine is an organophosphate herbicide. Used as a foliar spray for control and/or growth suppression of many woody plant species such as maple, birch, alder, blackberry, vine maple, ash, and oak. Susceptible treated plants normally fail to refoliate during the growing season following treatment and subsequently die. Fosamine functions as a plant growth regulator. It is sometimes referred to as a “dormancy enforcer,” but its specific mechanism of action has not been identified. There is some evidence that it inhibits mitosis in susceptible plants. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | - Amine
- Carbamate
- Ester
- Herbicide
- Organic Compound
- Organophosphate
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| DuPont krenite brush control agent | MeSH | | Fosamine monoammonium salt | MeSH | | Fosamine monolithium salt | MeSH | | Fosamine monosodium salt | MeSH | | Fosamine zinc (2:1) salt | MeSH | | Ammonium ethyl carbamoylphosphonate | MeSH | | Fosamine ammonium | MeSH | | Fosamine-ammonium | MeSH |
|
|---|
| Chemical Formula | C3H8NO4P |
|---|
| Average Molecular Mass | 153.074 g/mol |
|---|
| Monoisotopic Mass | 153.019 g/mol |
|---|
| CAS Registry Number | 59682-52-9 |
|---|
| IUPAC Name | carbamoyl(ethoxy)phosphinic acid |
|---|
| Traditional Name | fosamine |
|---|
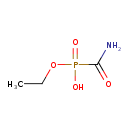
| SMILES | CCOP(O)(=O)C(N)=O |
|---|
| InChI Identifier | InChI=1S/C3H8NO4P/c1-2-8-9(6,7)3(4)5/h2H2,1H3,(H2,4,5)(H,6,7) |
|---|
| InChI Key | UCHDFLNGIZUADY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as organic phosphonic acids. These are organic compounds containing phosphonic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic phosphonic acids and derivatives |
|---|
| Sub Class | Organic phosphonic acids |
|---|
| Direct Parent | Organic phosphonic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organophosphonic acid
- Carboximidic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organophosphorus compound
- Organooxygen compound
- Organonitrogen compound
- Imine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-2900000000-9ddad6d9abffcf7ee27d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-9600000000-eb27e2b147af968a995d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-003r-9200000000-d69f49f92185a2e28ce5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-2900000000-8420f295de5d8bb0a242 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0adl-9800000000-c2a988dc2c60620ae19b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9400000000-38c9a5c534207ffca3fb | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Inhalation (1) ; oral (1); dermal (1) |
|---|
| Mechanism of Toxicity | Fosamine is an eye irritant. It likely binds to or modifies the TRPA1 protein which leads to a lachrymatory (tearing) response.
|
|---|
| Metabolism | Metabolism of organophosphates occurs principally by oxidation, and hydrolysis by esterases and by reaction with glutathione. Demethylation and glucuronidation may also occur. Oxidation of organophosphorus pesticides may result in moderately toxic products. In general, phosphorothioates are not directly toxic but require oxidative metabolism to the proximal toxin. The glutathione transferase reactions produce products that are, in most cases, of low toxicity. Fosamine rapidly passes through the body; elimination is primarily in the feces and less in the urine. It does not bioaccumulate (build up) in mammals. |
|---|
| Toxicity Values | The oral LD50 is 24,400 mg/kg for rats |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Used as a herbicide to control growth of woody plants. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Occupational exposure to fosamine occurs through dermal contact and inhalation of dust and sprays, especially to workers applying the compound as a herbicide. Fosamine has low to very low toxicity if individuals accidentally inhale or eat residues and has moderate toxicity if touched. It is not irritating to the eyes, but it can cause mild to moderate skin irritation. Fosamine can cause moderate eye injury or irritation. In one subchronic oral study, the laboratory animals given the highest dose exhibited some statistically significant effects, including effects to the kidneys, bladder and decreases in body weight. |
|---|
| Symptoms | Fosamine has low to very low toxicity if individuals accidentally inhale or eat residues and has moderate toxicity if touched. It is not irritating to the eyes, but it can cause mild to moderate skin irritation. Fosamine is not a skin sensitizer.
|
|---|
| Treatment | For acute exposures and first aid: EYES: irrigate opened eyes for several minutes under running water. INGESTION: do not induce vomiting. Rinse mouth with water (never give anything by mouth to an unconscious person). Seek immediate medical advice. SKIN: should be treated immediately by rinsing the affected parts in cold running water for at least 15 minutes, followed by thorough washing with soap and water. If necessary, the person should shower and change contaminated clothing and shoes, and then must seek medical attention. INHALATION: supply fresh air. If required provide artificial respiration. |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 33257 |
|---|
| Kegg Compound ID | C18789 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|