| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 13:50:53 UTC |
|---|
| Update Date | 2016-11-09 01:23:26 UTC |
|---|
| Accession Number | CHEM045952 |
|---|
| Identification |
|---|
| Common Name | Maprotiline, Desmethyl |
|---|
| Class | Small Molecule |
|---|
| Description | demethylmaprotiline is a metabolite of maprotiline. Maprotiline (sold as Deprilept, Ludiomil, Psymion) is a tetracyclic antidepressant (TeCA). However, Maprotiline's fourth ring is spurious, as formed by a bridge across the central tricyclic ring. It is a strong norepinephrine reuptake inhibitor with only weak effects on serotonin and dopamine reuptake. (Wikipedia) |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 9,10-Ethanoanthracene-9(10H)-propylamine | HMDB |
|
|---|
| Chemical Formula | C19H21N |
|---|
| Average Molecular Mass | 263.377 g/mol |
|---|
| Monoisotopic Mass | 263.167 g/mol |
|---|
| CAS Registry Number | 5721-37-9 |
|---|
| IUPAC Name | 3-{tetracyclo[6.6.2.0²,⁷.0⁹,¹⁴]hexadeca-2,4,6,9,11,13-hexaen-1-yl}propan-1-amine |
|---|
| Traditional Name | 3-{tetracyclo[6.6.2.0²,⁷.0⁹,¹⁴]hexadeca-2,4,6,9,11,13-hexaen-1-yl}propan-1-amine |
|---|
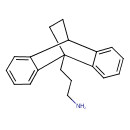
| SMILES | NCCCC12CCC(C3=CC=CC=C13)C1=CC=CC=C21 |
|---|
| InChI Identifier | InChI=1S/C19H21N/c20-13-5-11-19-12-10-14(15-6-1-3-8-17(15)19)16-7-2-4-9-18(16)19/h1-4,6-9,14H,5,10-13,20H2 |
|---|
| InChI Key | IFHUOEQJTQWFGJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as anthracenes. These are organic compounds containing a system of three linearly fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Anthracenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Anthracenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Anthracene
- Tetralin
- Aralkylamine
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Primary amine
- Organonitrogen compound
- Primary aliphatic amine
- Amine
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-053r-9080000000-12ddba41c4c4cee9f454 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dj-0090000000-e3cf814b95b418ce270c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ot-0090000000-98929235ad1abaa60b3e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066u-5590000000-cdd9a806a71ed0783368 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-2cfe1fcdcee2b938ffd1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0090000000-e3b2f824800d5a86f61c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0apj-2090000000-988a143a12803492905e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-330f29be739abf456d25 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0090000000-d89ebdfa727b727f5887 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-0090000000-802b45c948e9f2a77ba7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dj-0090000000-1a23789291e198a78c4a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ot-0090000000-88c2720aa68a8b184f9c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066u-0190000000-16c0738d2febf5623de6 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0060993 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 106651 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 2734 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|