| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 13:49:09 UTC |
|---|
| Update Date | 2016-11-09 01:23:26 UTC |
|---|
| Accession Number | CHEM045922 |
|---|
| Identification |
|---|
| Common Name | 2,5-Dimethoxy-4-Iodoamfetamine (DOI) |
|---|
| Class | Small Molecule |
|---|
| Description | An organoiodine compound that is amphetamine bearing two methoxy substituents at positions 2 and 5 as well as an iodo substituent at position 4. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
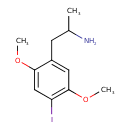
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane | ChEBI | | 1-(4-Iodo-2,5-dimethoxyphenyl)-2-aminopropane | ChEBI | | 2,5-Dimethoxy-4-iodoamphetamine | ChEBI | | 2,5-Dimethoxy-4-iodophenylisopropylamine | ChEBI | | 4-DOI | ChEBI | | 4-Iodo-2,5-dimethoxyphenylisopropylamine | ChEBI | | 2,5-Dimethoxy-4-iodoamphetamine hydrochloride | MeSH, HMDB | | 4-iodo-2,5-Dimethoxyphenylisopropylamine hydrochloride, (+-)-isomer | MeSH, HMDB | | 4-iodo-2,5-Dimethoxyphenylisopropylamine hydrochloride, (R)-isomer | MeSH, HMDB | | 4-iodo-2,5-Dimethoxyphenylisopropylamine, (+-)-isomer | MeSH, HMDB | | 4-iodo-2,5-Dimethoxyphenylisopropylamine, (R)-isomer | MeSH, HMDB | | 4-iodo-2,5-Dimethoxyphenylisopropylamine, 123I-labeled | MeSH, HMDB | | 4-iodo-2,5-Dimethoxyphenylisopropylamine, 131I-labeled | MeSH, HMDB | | DOI CPD | MeSH, HMDB | | DOI-P | MeSH, HMDB |
|
|---|
| Chemical Formula | C11H16INO2 |
|---|
| Average Molecular Mass | 321.158 g/mol |
|---|
| Monoisotopic Mass | 321.023 g/mol |
|---|
| CAS Registry Number | 82864-02-6 |
|---|
| IUPAC Name | 1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine |
|---|
| Traditional Name | 1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine |
|---|
| SMILES | COC1=CC(I)=C(OC)C=C1CC(C)N |
|---|
| InChI Identifier | InChI=1S/C11H16INO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3 |
|---|
| InChI Key | BGMZUEKZENQUJY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as amphetamines and derivatives. These are organic compounds containing or derived from 1-phenylpropan-2-amine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenethylamines |
|---|
| Direct Parent | Amphetamines and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Amphetamine or derivatives
- P-dimethoxybenzene
- Dimethoxybenzene
- Phenylpropane
- Phenoxy compound
- Anisole
- Methoxybenzene
- Phenol ether
- Alkyl aryl ether
- Halobenzene
- Aralkylamine
- Iodobenzene
- Aryl halide
- Aryl iodide
- Ether
- Organoiodide
- Organohalogen compound
- Primary aliphatic amine
- Primary amine
- Hydrocarbon derivative
- Amine
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organonitrogen compound
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9164000000-8670b9e41db86f9e5647 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05fr-0009000000-7e1b709f08f2b8b4e341 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ab9-1039000000-b428da251f63657d2e7a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05gu-8090000000-63d10d52bd6880b50b4f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0009000000-f472942727229979f72c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fk9-0129000000-d9ab02ebceb94dcffa49 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0079-3492000000-f6b5a951bef941808ba5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05fr-0009000000-ad3ec49d3e0823f30c05 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fr-0049000000-e127d15849f12aafcbdd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004l-7290000000-8141c19f7e7ad8a420c6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0069000000-7fbfba80a34bab33c742 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0689000000-62617a414351d313825d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0920000000-382457d1193d6d3250d7 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0260203 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 1192 |
|---|
| ChEBI ID | 64629 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|