| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 13:47:53 UTC |
|---|
| Update Date | 2016-11-09 01:23:26 UTC |
|---|
| Accession Number | CHEM045897 |
|---|
| Identification |
|---|
| Common Name | 2,5-Dimethoxy-4-Methylphenethylamine (2C-D) |
|---|
| Class | Small Molecule |
|---|
| Description | 2C-D (2,5-dimethoxy-4-methylphenethylamine or 2C-M) is a psychedelic drug of the 2C family that is sometimes used as an entheogen. It was first synthesized in 1970 by a team from the Texas Research Institute of Mental Sciences, and its activity was subsequently investigated in humans by Alexander Shulgin. In his book PiHKAL, Shulgin lists the dosage range as being from 20 to 60 mg.Not much information is known about the toxicity of 2C-D, as no major studies have been conducted. According to Shulgin, the effects of 2C-D typically last for 4–6 hours. Shulgin himself referred to this substance as a “pharmacological tofu,” meaning that when mixed with other substances, it can extend or potentiate their effects without coloring the experience too much, in a manner similar to how tofu absorbs the flavors of sauces or spices it is cooked with. Hanscarl Leuner, working in Germany, explored the use of 2C-D under the name LE-25 in psychotherapeutic research. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
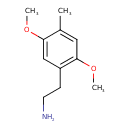
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,5-Dimethoxy-4-methyl-beta-phenethylamine | MeSH | | 2,5-DMP CPD | MeSH |
|
|---|
| Chemical Formula | C11H17NO2 |
|---|
| Average Molecular Mass | 195.262 g/mol |
|---|
| Monoisotopic Mass | 195.126 g/mol |
|---|
| CAS Registry Number | 24333-19-5 |
|---|
| IUPAC Name | 2-(2,5-dimethoxy-4-methylphenyl)ethan-1-amine |
|---|
| Traditional Name | 2-(2,5-dimethoxy-4-methylphenyl)ethanamine |
|---|
| SMILES | COC1=CC(CCN)=C(OC)C=C1C |
|---|
| InChI Identifier | InChI=1S/C11H17NO2/c1-8-6-11(14-3)9(4-5-12)7-10(8)13-2/h6-7H,4-5,12H2,1-3H3 |
|---|
| InChI Key | UNQQFDCVEMVQHM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dimethoxybenzenes. These are organic aromatic compounds containing a monocyclic benzene moiety carrying exactly two methoxy groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Methoxybenzenes |
|---|
| Direct Parent | Dimethoxybenzenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - P-dimethoxybenzene
- Dimethoxybenzene
- Phenethylamine
- Phenoxy compound
- Anisole
- 2-arylethylamine
- Phenol ether
- Alkyl aryl ether
- Aralkylamine
- Toluene
- Ether
- Organic nitrogen compound
- Primary amine
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Organopnictogen compound
- Organic oxygen compound
- Amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0900000000-359f4a617a7c97a70b57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004j-1900000000-ab4ff2b6e4a9d690f729 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00rj-8900000000-873665b622ccc54d367d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-1f680010193119590444 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0900000000-647b4ef651f883f9409a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fft-3900000000-5c145872a0f4761c93a4 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | 2C-D |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 135740 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|