| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 13:34:00 UTC |
|---|
| Update Date | 2016-11-09 01:23:23 UTC |
|---|
| Accession Number | CHEM045665 |
|---|
| Identification |
|---|
| Common Name | tianeptine |
|---|
| Class | Small Molecule |
|---|
| Description | Tianeptine is a drug used primarily in the treatment of major depressive disorder and has been studied in the treatment of irritable bowel syndrome (IBS) [A31983]. Structurally, it is classified as a tricyclic antidepressant (TCA), however, it possesses different pharmacological properties than typical tricyclic antidepressants [A31969].
Tianeptine was discovered and patented by The French Society of Medical Research in the 1960s [L2946]. Currently, tianeptine is approved in France and manufactured and marketed by Laboratories Servier SA; it is also marketed in several other European countries under the trade name “Coaxil” as well as in Asia (including Singapore) and Latin America as “Stablon” and “Tatinol” but it is not available in Australia, Canada, New Zealand, the U.K. or the U.S. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
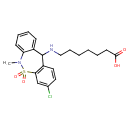
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Stablon | Kegg | | Ardix brand OF tianeptine, monosodium salt | HMDB | | Tianeptine, (+-)-isomer | HMDB | | Tianeptine, monosodium salt, (+-)-isomer | HMDB | | (3-Chloro-6-methyl-5,5-dioxo-6,11-dihydrodibenzo(c,F)(1,2)thiazepin-11-yl)-7-aminoheptanoic acid | HMDB | | Coaxil | HMDB | | Tianeptine, monosodium salt | HMDB |
|
|---|
| Chemical Formula | C21H25ClN2O4S |
|---|
| Average Molecular Mass | 436.952 g/mol |
|---|
| Monoisotopic Mass | 436.122 g/mol |
|---|
| CAS Registry Number | 66981-73-5 |
|---|
| IUPAC Name | 7-({6-chloro-10-methyl-9,9-dioxo-9λ⁶-thia-10-azatricyclo[9.4.0.0³,⁸]pentadeca-1(15),3(8),4,6,11,13-hexaen-2-yl}amino)heptanoic acid |
|---|
| Traditional Name | tianeptine |
|---|
| SMILES | CN1C2=CC=CC=C2C(NCCCCCCC(O)=O)C2=C(C=C(Cl)C=C2)S1(=O)=O |
|---|
| InChI Identifier | InChI=1S/C21H25ClN2O4S/c1-24-18-9-6-5-8-16(18)21(23-13-7-3-2-4-10-20(25)26)17-12-11-15(22)14-19(17)29(24,27)28/h5-6,8-9,11-12,14,21,23H,2-4,7,10,13H2,1H3,(H,25,26) |
|---|
| InChI Key | JICJBGPOMZQUBB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as medium-chain fatty acids. These are fatty acids with an aliphatic tail that contains between 4 and 12 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Medium-chain fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Medium-chain fatty acid
- Halogenated fatty acid
- Heterocyclic fatty acid
- Aralkylamine
- Aryl chloride
- Aryl halide
- Benzenoid
- Organosulfonic acid amide
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Amino acid or derivatives
- Amino acid
- Carboxylic acid derivative
- Carboxylic acid
- Secondary aliphatic amine
- Monocarboxylic acid or derivatives
- Secondary amine
- Azacycle
- Organoheterocyclic compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic nitrogen compound
- Organohalogen compound
- Organochloride
- Organonitrogen compound
- Organooxygen compound
- Organopnictogen compound
- Carbonyl group
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0297-2955000000-f5d01fbffed94ca1d3d4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0109-8938200000-3888305fcc28fb79c75d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0102900000-b2120851dc0c11f1c49c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-1139100000-82f01656084a2a95b7d9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0apl-9103000000-0ce94a818793b81c35d9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0002900000-a92759de6778d9d58e87 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-1107900000-7a69b9c7b39711b06f2a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9421000000-97f7c1339139f4d31260 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000900000-52ebdf5ff9776f6b3b82 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000l-1009600000-85329350581bdd148498 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-2095000000-be5b486ec2b08095f0af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000l-0050900000-330adb08d607196bfb09 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0091200000-ec4022ca8e157a7fea26 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-1192000000-af153fd72bc53018e74a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB09289 |
|---|
| HMDB ID | HMDB0042038 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Tianeptine |
|---|
| Chemspider ID | 62102 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 68870 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|