| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 13:32:34 UTC |
|---|
| Update Date | 2016-11-09 01:23:23 UTC |
|---|
| Accession Number | CHEM045637 |
|---|
| Identification |
|---|
| Common Name | trofosamide |
|---|
| Class | Small Molecule |
|---|
| Description | Trofosfamide has been used in trials studying the treatment of Ependymomas, Medulloblastomas, Sarcoma, Soft Tissue, Supratentorial PNETs, and Recurrent Brain Tumors. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
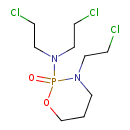
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Z-4828a-4828Trophosphamide | ChEMBL | | Ixoten | MeSH | | N,N,N'-tris(2-chloroethyl)-n'-O-propylene phosphoric acid ester diamide | MeSH | | Trophosphamide | MeSH | | Genoxal trofosfamida | MeSH |
|
|---|
| Chemical Formula | C9H18Cl3N2O2P |
|---|
| Average Molecular Mass | 323.580 g/mol |
|---|
| Monoisotopic Mass | 322.017 g/mol |
|---|
| CAS Registry Number | 22089-22-1 |
|---|
| IUPAC Name | 2-[bis(2-chloroethyl)amino]-3-(2-chloroethyl)-1,3,2lambda5-oxazaphosphinan-2-one |
|---|
| Traditional Name | 2-[bis(2-chloroethyl)amino]-3-(2-chloroethyl)-1,3,2lambda5-oxazaphosphinan-2-one |
|---|
| SMILES | ClCCN(CCCl)P1(=O)OCCCN1CCCl |
|---|
| InChI Identifier | InChI=1S/C9H18Cl3N2O2P/c10-2-6-13-5-1-9-16-17(13,15)14(7-3-11)8-4-12/h1-9H2 |
|---|
| InChI Key | UMKFEPPTGMDVMI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as isofamides. These are oxazaphospholanes containing the isofamide skeleton. Isofamide is a heterocyclic compound made up of a 1,3,2-oxazaphospholane, where the phosphorus atom is part of a phosphodiamide group, and the oxazaphospholane is substituted by two haloalkyl chains. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Oxazaphosphinanes |
|---|
| Sub Class | Isofamides |
|---|
| Direct Parent | Isofamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Isofamide
- Nitrogen mustard
- Phosphoric monoester diamide
- Organic phosphoric acid derivative
- Organic phosphoric acid amide
- Azacycle
- Oxacycle
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organochloride
- Organopnictogen compound
- Organohalogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Alkyl halide
- Alkyl chloride
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01wr-1390000000-0afd21ebf3af426e20d9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-9323000000-c915e6f1b855e99ba0a4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-4900000000-606f1434f3fb12b4b398 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01r6-9100000000-fdd511c33c062a620634 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4j-1390000000-1f0c252d058b66c4b0b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0159-7900000000-1cee7f661aec43a3eb62 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-5910000000-7fe3392edc1195eb4525 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB12902 |
|---|
| HMDB ID | HMDB0259291 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Trofosfamide |
|---|
| Chemspider ID | 59129 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|