| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 13:24:27 UTC |
|---|
| Update Date | 2016-11-09 01:23:21 UTC |
|---|
| Accession Number | CHEM045538 |
|---|
| Identification |
|---|
| Common Name | 3-[[2-(acetylamino)-4-aminophenyl]azo]naphthalene-1,5-disulfonic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
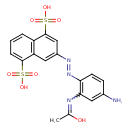
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N-{5-amino-2-[(e)-2-(4,8-disulfonaphthalen-2-yl)diazen-1-yl]phenyl}ethanimidate | Generator | | N-{5-amino-2-[(e)-2-(4,8-disulphonaphthalen-2-yl)diazen-1-yl]phenyl}ethanimidate | Generator | | N-{5-amino-2-[(e)-2-(4,8-disulphonaphthalen-2-yl)diazen-1-yl]phenyl}ethanimidic acid | Generator |
|
|---|
| Chemical Formula | C18H16N4O7S2 |
|---|
| Average Molecular Mass | 464.470 g/mol |
|---|
| Monoisotopic Mass | 464.046 g/mol |
|---|
| CAS Registry Number | 117-88-4 |
|---|
| IUPAC Name | N-{5-amino-2-[(E)-2-(4,8-disulfonaphthalen-2-yl)diazen-1-yl]phenyl}ethanimidic acid |
|---|
| Traditional Name | N-{5-amino-2-[(E)-2-(4,8-disulfonaphthalen-2-yl)diazen-1-yl]phenyl}ethanimidic acid |
|---|
| SMILES | CC(O)=NC1=C(C=CC(N)=C1)\N=N\C1=CC2=C(C=CC=C2S(O)(=O)=O)C(=C1)S(O)(=O)=O |
|---|
| InChI Identifier | InChI=1S/C18H16N4O7S2/c1-10(23)20-16-7-11(19)5-6-15(16)22-21-12-8-14-13(18(9-12)31(27,28)29)3-2-4-17(14)30(24,25)26/h2-9H,19H2,1H3,(H,20,23)(H,24,25,26)(H,27,28,29)/b22-21+ |
|---|
| InChI Key | KOXYXBGVZGWXED-QURGRASLSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1-naphthalene sulfonates. These are organic aromatic compounds that contain a naphthalene moiety that carries a sulfonic acid group at the 1-position. Naphthalene is a bicyclic compound that is made up of two fused benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Naphthalenes |
|---|
| Sub Class | Naphthalene sulfonic acids and derivatives |
|---|
| Direct Parent | 1-naphthalene sulfonates |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-naphthalene sulfonic acid or derivatives
- 1-naphthalene sulfonate
- Acetanilide
- Arylsulfonic acid or derivatives
- N-acetylarylamine
- 1-sulfo,2-unsubstituted aromatic compound
- Anilide
- N-arylamide
- Aniline or substituted anilines
- Monocyclic benzene moiety
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Organosulfonic acid
- Sulfonyl
- Acetamide
- Amino acid or derivatives
- Azo compound
- Secondary carboxylic acid amide
- Carboxamide group
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Carboxylic acid derivative
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Organosulfur compound
- Primary amine
- Carbonyl group
- Organopnictogen compound
- Amine
- Organic oxide
- Hydrocarbon derivative
- Organic oxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kb-0211900000-16c64471c7427691b8a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ac0-0215900000-e7bf145d711ccc2fe936 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-059b-0955000000-c3f1ac80d073bbadca5e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-1101900000-3a9c1b5ba1e9e035ec05 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01x0-5504900000-083358bef64d44d2fed5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0230-5900000000-ca118c249245dacb0300 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|