| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 12:55:52 UTC |
|---|
| Update Date | 2016-11-09 01:23:18 UTC |
|---|
| Accession Number | CHEM045201 |
|---|
| Identification |
|---|
| Common Name | lanreotide |
|---|
| Class | Small Molecule |
|---|
| Description | Lanreotide is a drug employed in the management of acromegaly (a hormonal condition caused by excess growth hormone) in addition to symptoms caused by neuroendocrine tumors, especially carcinoid syndrome. This drug is a long-acting analog of the drug somatostatin, a growth hormone inhibitor. Lanreotide is manufactured by the company, _Ipsen Pharmaceuticals_ as lanreotide acetate, and marketed as _Somatuline_. It is approved in several countries worldwide, including the United Kingdom, Australia, and Canada. Lanreotide was first approved for use in the United States by the FDA on August 30, 2007. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
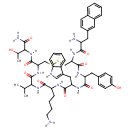
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Somatulina | MeSH | | Somatuline | MeSH | | L-Threoninamide, 3-(2-naphthalenyl)-D-alanyl-L-cysteinyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-valyl-L-cysteinyl-, cyclic (2-7)-disulfide | MeSH | | Lanreotide acetate | MeSH | | Nal-cyclo(cys-tyr-TRP-lys-val-cys)THR-NH2 | MeSH | | Lanreotide-SR | MeSH | | 2-Naphthylalanyl-cyclo(cysteinyl-tyrosyl-tryptophyl-lysyl-valyl-cysteinyl)-threoninamide | MeSH | | Naphthyl-cyclo(cys-tyr-TRP-lys-val-cys)THR-NH2 | MeSH | | Nal-cyclo(cys-tyr-TRP-lys-val-cys)-THR-NH2 | MeSH | | Somatulin | MeSH | | 188Re-Lanreotide | MeSH | | Naphthalenyl-cyclo(cysteinyl-tyrosyl-tryptophyl-lysyl-valyl-cysteinyl)threoninamide | MeSH | | Lanreotide | MeSH | | Lanreotide acetic acid | Generator | | 2-{[(19-{[2-amino-1-hydroxy-3-(naphthalen-2-yl)propylidene]amino}-10-(4-aminobutyl)-6,9,12,15,18-pentahydroxy-16-[(4-hydroxyphenyl)methyl]-13-[(1H-indol-3-yl)methyl]-7-(propan-2-yl)-1,2-dithia-5,8,11,14,17-pentaazacycloicosa-5,8,11,14,17-pentaen-4-yl)(hydroxy)methylidene]amino}-3-hydroxybutanimidate | Generator |
|
|---|
| Chemical Formula | C54H69N11O10S2 |
|---|
| Average Molecular Mass | 1096.330 g/mol |
|---|
| Monoisotopic Mass | 1095.467 g/mol |
|---|
| CAS Registry Number | 108736-35-2 |
|---|
| IUPAC Name | 2-({19-[2-amino-3-(naphthalen-2-yl)propanamido]-10-(4-aminobutyl)-16-[(4-hydroxyphenyl)methyl]-13-[(1H-indol-3-yl)methyl]-6,9,12,15,18-pentaoxo-7-(propan-2-yl)-1,2-dithia-5,8,11,14,17-pentaazacycloicosan-4-yl}formamido)-3-hydroxybutanamide |
|---|
| Traditional Name | 2-({19-[2-amino-3-(naphthalen-2-yl)propanamido]-10-(4-aminobutyl)-16-[(4-hydroxyphenyl)methyl]-13-(1H-indol-3-ylmethyl)-7-isopropyl-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosan-4-yl}formamido)-3-hydroxybutanamide |
|---|
| SMILES | [H]N([H])CCCCC1N([H])C(=O)C(CC2=CN([H])C3=CC=CC=C23)N([H])C(=O)C(CC2=CC=C(O)C=C2)N([H])C(=O)C(CSSCC(N([H])C(=O)C(N([H])C1=O)C(C)C)C(=O)N([H])C(C(C)O)C(=O)N([H])[H])N([H])C(=O)C(CC1=CC2=CC=CC=C2C=C1)N([H])[H] |
|---|
| InChI Identifier | InChI=1S/C54H69N11O10S2/c1-29(2)45-54(75)63-44(53(74)65-46(30(3)66)47(57)68)28-77-76-27-43(62-48(69)38(56)23-32-15-18-33-10-4-5-11-34(33)22-32)52(73)60-41(24-31-16-19-36(67)20-17-31)50(71)61-42(25-35-26-58-39-13-7-6-12-37(35)39)51(72)59-40(49(70)64-45)14-8-9-21-55/h4-7,10-13,15-20,22,26,29-30,38,40-46,58,66-67H,8-9,14,21,23-25,27-28,55-56H2,1-3H3,(H2,57,68)(H,59,72)(H,60,73)(H,61,71)(H,62,69)(H,63,75)(H,64,70)(H,65,74) |
|---|
| InChI Key | PUDHBTGHUJUUFI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acylaminobenzoic acid and derivatives. These are derivatives of amino benzoic acid derivatives where the amine group is N-acylated. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Acylaminobenzoic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acylaminobenzoic acid or derivatives
- 2-halobenzoic acid or derivatives
- 4-halobenzoic acid or derivatives
- Halobenzoic acid or derivatives
- Benzamide
- Anilide
- Benzoyl
- Iodobenzene
- Halobenzene
- Aryl halide
- Aryl iodide
- 1,3-dicarbonyl compound
- Tertiary carboxylic acid amide
- Vinylogous halide
- Secondary carboxylic acid amide
- Carboxamide group
- Secondary alcohol
- Carboxylic acid derivative
- Polyol
- Organoiodide
- Organohalogen compound
- Primary alcohol
- Alcohol
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-006t-4340104090-6be3ea865b49f29210e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0v4i-7900000123-eaa594f5bcf0aa756ded | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-1965373210-454c2d978a738c9cc3e0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014j-9070101161-e3b5852fe384fea43816 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03fu-9010100041-0492cc7b9a68886e29dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-4902423210-e1413c69ee0448130ed1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-9100000000-3a898a1e589d05e993ab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-005a-9300000013-177d2fa12fa34fd00eca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000x-3900000010-7b8292d3e5f67208916a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-9000000001-3848eb09dcc7e8c585a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9510000004-df04c45dfb4d319d5344 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f96-1900000010-a6a2ca06bcafbc525c72 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB06791 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Lanreotide |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 71349 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|