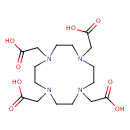
| Synonyms | | Value | Source |
|---|
| 1,4,7,10-Dota | ChEBI | | 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid | ChEBI | | 1,4,7,10-Tetraazacyclododecane-N,n',n'',n'''-tetraacetic acid | ChEBI | | 2,2',2',2'''-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetryl)tetraacetic acid | ChEBI | | DOTA acid | ChEBI | | Tetraxetan | ChEBI | | Tetraxetanum | ChEBI | | 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetate | Generator | | 1,4,7,10-Tetraazacyclododecane-N,n',n'',n'''-tetraacetate | Generator | | 2,2',2',2'''-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetryl)tetraacetate | Generator | | 1,4,7,10-Tetraazacyclododecane- 1,4,7,10-tetraacetic acid | MeSH | | 1,4,7,10-Tetraazacyclododecane--N,n',n'',n'''-tetraacetic acid | MeSH | | 1,4,7,10-Tetrakis(carboxymethyl)-1,4,7,10-tetraazacyclododecane | MeSH | | 2,2',2'',2'''-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetryl)tetraacetic acid | MeSH | | CuDOTA | MeSH | | ,4,7,10-Tetraazacyclododecane-N,n',n'',n'''-tetraacetic acid | ChEBI | | ,4,7,10-Tetraazacyclododecane-N,n',n'',n'''-tetraacetate | Generator |
|
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052f-7009100000-113938c8a63c42696f4b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_4_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_4_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0009600000-a3d9ec8c18fc0d095c05 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0009100000-3e2b970e52d33f5cedb5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-0009000000-1f14bed5e34496ca6bb2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0003900000-291594c4a8457960bed6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0006900000-fa9da763c0d3786d373d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-06r6-9005200000-09040057f119171791f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000900000-87f2dd104cebcd6b3f78 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0001900000-652fffe38df245af62de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ufs-0059000000-3cfcfb6ca52ac93950f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0002900000-1d8bb7296d13248128d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pvi-0029200000-6ffc3f7749cc43954fdb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052b-0059000000-5c9354194470d1901e58 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|