| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 12:09:57 UTC |
|---|
| Update Date | 2016-11-09 01:23:07 UTC |
|---|
| Accession Number | CHEM044393 |
|---|
| Identification |
|---|
| Common Name | D-N-(4-hydroxyphenyl)glycine |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
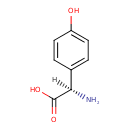
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (R)-alpha-Amino-4-hydroxybenzeneacetic acid | ChEBI | | 4-HYDROXYPHENYLGLYCINE | ChEBI | | D-N-(4-Hydroxyphenyl)glycine | ChEBI | | (AlphaR)-alpha-amino-4-hydroxybenzeneacetic acid | Kegg | | (R)-a-Amino-4-hydroxybenzeneacetate | Generator | | (R)-a-Amino-4-hydroxybenzeneacetic acid | Generator | | (R)-alpha-Amino-4-hydroxybenzeneacetate | Generator | | (R)-Α-amino-4-hydroxybenzeneacetate | Generator | | (R)-Α-amino-4-hydroxybenzeneacetic acid | Generator | | (AlphaR)-a-amino-4-hydroxybenzeneacetate | Generator | | (AlphaR)-a-amino-4-hydroxybenzeneacetic acid | Generator | | (AlphaR)-alpha-amino-4-hydroxybenzeneacetate | Generator | | (AlphaR)-α-amino-4-hydroxybenzeneacetate | Generator | | (AlphaR)-α-amino-4-hydroxybenzeneacetic acid | Generator | | (2R)-amino(4-Hydroxyphenyl)ethanoate | Generator | | 4-Hydroxyphenylglycine, (R)-isomer | MeSH | | 4-Hydroxyphenylglycine, 4-methylbenzenesulfonate, (+-)-isomer | MeSH | | 4-Hydroxyphenylglycine, monosodium salt | MeSH | | (R,S)-3HPG | MeSH | | 4-Hydroxyphenylglycine hydrochloride, (R)-isomer | MeSH | | 4-Hydroxyphenylglycine, (+-)-isomer | MeSH | | D-P-Hydroxyphenylglycine | MeSH | | Oxfenicine | MeSH | | P-Hydroxyphenylglycine | MeSH | | 4-Hydroxyphenylglycine hydrobromide, (+-)-isomer | MeSH | | 4-Hydroxyphenylglycine perchlorate, (+-)-isomer | MeSH | | 4-Hydroxyphenylglycine, (S)-isomer | MeSH | | 4-Hydroxyphenylglycine, 2,4-dimethylbenzenesulfonate, (+-)-isomer | MeSH | | 4-Hydroxyphenylglycine, 4-methylbenzenesulfonate, (S)-isomer | MeSH | | 4-Hydroxyphenylglycine, monosodium salt, (R)-isomer | MeSH | | L-4-Hydroxyphenylglycine | MeSH | | 4-Hydroxyphenylglycine, 2,4-dimethylbenzenesulfonate, (R)-isomer | MeSH |
|
|---|
| Chemical Formula | C8H9NO3 |
|---|
| Average Molecular Mass | 167.162 g/mol |
|---|
| Monoisotopic Mass | 167.058 g/mol |
|---|
| CAS Registry Number | 22818-40-2 |
|---|
| IUPAC Name | (2R)-2-amino-2-(4-hydroxyphenyl)acetic acid |
|---|
| Traditional Name | D-4-hydroxyphenylglycine |
|---|
| SMILES | [H][C@](N)(C(O)=O)C1=CC=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C8H9NO3/c9-7(8(11)12)5-1-3-6(10)4-2-5/h1-4,7,10H,9H2,(H,11,12)/t7-/m1/s1 |
|---|
| InChI Key | LJCWONGJFPCTTL-SSDOTTSWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as d-alpha-amino acids. These are alpha amino acids which have the D-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | D-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - D-alpha-amino acid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Aralkylamine
- Monocyclic benzene moiety
- Benzenoid
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Amine
- Hydrocarbon derivative
- Primary amine
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Primary aliphatic amine
- Organic oxygen compound
- Carbonyl group
- Organic nitrogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-7900000000-b5749f79598d6ae4f70b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-73882345716a5f7f7dea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-2900000000-38465b6035f4c1628c0a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-9200000000-86f53fd8d6118376d181 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00xr-0900000000-0dec5db52da8993b438e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-1900000000-7ae7e09e4ea996a336cc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fxy-9500000000-1f01fba0bd30ed70f5fb | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB04308 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | D-4-HYDROXYPHENYLGLYCINE |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 15695 |
|---|
| PubChem Compound ID | 89853 |
|---|
| Kegg Compound ID | C03493 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|