| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 11:59:12 UTC |
|---|
| Update Date | 2016-11-09 01:23:05 UTC |
|---|
| Accession Number | CHEM044273 |
|---|
| Identification |
|---|
| Common Name | ethisterone |
|---|
| Class | Small Molecule |
|---|
| Description | A 17beta-hydroxy steroid that is testosterone in which the 17beta hydrogen is replaced by an ethynyl group. Ethisterone was the first orally active progestin and is a metabolite of danazol. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
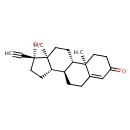
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 17-beta-Hydroxy-17-alpha-ethynyl-4-androsten-3-one | ChEBI | | 17-Ethynyl-17beta-hydroxyandrost-4-en-3-one | ChEBI | | 17-Hydroxy-17alpha-pregn-4-en-20-yn-3-one | ChEBI | | 17alpha-Ethinyltestosterone | ChEBI | | 17alpha-Ethynyl-17beta-hydroxyandrost-4-en-3-one | ChEBI | | 17alpha-Ethynyltestosterone | ChEBI | | Aethisteron | ChEBI | | Aethisteronum | ChEBI | | Anhydrohydroxyprogesterone | ChEBI | | Anhydroxyprogesterone | ChEBI | | Ethisteronum | ChEBI | | Ethynyltestosterone | ChEBI | | Etisterona | ChEBI | | Lutocylol | ChEBI | | Nugestoral | ChEBI | | Ora-lutin | ChEBI | | Pranone | ChEBI | | Prodroxan | ChEBI | | Produxan | ChEBI | | Progestab | ChEBI | | Progestoral | ChEBI | | Syngestrotabs | ChEBI | | Trosinone | ChEBI | | 17alpha-Ethinyl testosterone | Kegg | | Progestolets | Kegg | | 17-b-Hydroxy-17-a-ethynyl-4-androsten-3-one | Generator | | 17-Β-hydroxy-17-α-ethynyl-4-androsten-3-one | Generator | | 17-Ethynyl-17b-hydroxyandrost-4-en-3-one | Generator | | 17-Ethynyl-17β-hydroxyandrost-4-en-3-one | Generator | | 17-Hydroxy-17a-pregn-4-en-20-yn-3-one | Generator | | 17-Hydroxy-17α-pregn-4-en-20-yn-3-one | Generator | | 17a-Ethinyltestosterone | Generator | | 17Α-ethinyltestosterone | Generator | | 17a-Ethynyl-17b-hydroxyandrost-4-en-3-one | Generator | | 17Α-ethynyl-17β-hydroxyandrost-4-en-3-one | Generator | | 17a-Ethynyltestosterone | Generator | | 17Α-ethynyltestosterone | Generator | | 17a-Ethinyl testosterone | Generator | | 17Α-ethinyl testosterone | Generator | | 17 alpha Ethynyltestosterone | HMDB | | 17 alpha-Ethynyltestosterone | HMDB | | Pregneninolone | HMDB |
|
|---|
| Chemical Formula | C21H28O2 |
|---|
| Average Molecular Mass | 312.446 g/mol |
|---|
| Monoisotopic Mass | 312.209 g/mol |
|---|
| CAS Registry Number | 434-03-7 |
|---|
| IUPAC Name | (1S,2R,10R,11S,14R,15S)-14-ethynyl-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-5-one |
|---|
| Traditional Name | ethisterone |
|---|
| SMILES | C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CC[C@@]2(O)C#C |
|---|
| InChI Identifier | InChI=1S/C21H28O2/c1-4-21(23)12-9-18-16-6-5-14-13-15(22)7-10-19(14,2)17(16)8-11-20(18,21)3/h1,13,16-18,23H,5-12H2,2-3H3/t16-,17+,18+,19+,20+,21+/m1/s1 |
|---|
| InChI Key | CHNXZKVNWQUJIB-CEGNMAFCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as androgens and derivatives. These are 3-hydroxylated C19 steroid hormones. They are known to favor the development of masculine characteristics. They also show profound effects on scalp and body hair in humans. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Androstane steroids |
|---|
| Direct Parent | Androgens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Androgen-skeleton
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- 17-hydroxysteroid
- Oxosteroid
- Hydroxysteroid
- Delta-4-steroid
- Cyclohexenone
- Ynone
- Tertiary alcohol
- Cyclic alcohol
- Cyclic ketone
- Ketone
- Acetylide
- Organic oxygen compound
- Organooxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Alcohol
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-0490000000-70da7a36790d59e5aec1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0aou-1259000000-15d6bab9887e263c6273 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0169000000-c3a3859c9249ba72e662 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0btj-0291000000-04631559fd3d6eeaf96d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0v4r-2490000000-b96a17af7ffa84b61a5e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-714673f1ccbbef0d107a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0039000000-edc9d17d6776c137fae1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052g-0090000000-09a994bdce08144c9cb6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0292-0092000000-87469d8eaf22bca0423c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01dj-0950000000-872492e74ef79726b1fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00xr-3940000000-1fb184dfb1c47b20c927 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-152b659b4891e36b54f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0009000000-2fd97457f6fe96a074b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a59-0394000000-326a8e9cae724f94ba3a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0060580 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Ethisterone |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 34749 |
|---|
| PubChem Compound ID | 5284557 |
|---|
| Kegg Compound ID | C14487 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|