| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 11:58:53 UTC |
|---|
| Update Date | 2016-11-09 01:23:05 UTC |
|---|
| Accession Number | CHEM044268 |
|---|
| Identification |
|---|
| Common Name | diethyldithiocarbaminic acid |
|---|
| Class | Small Molecule |
|---|
| Description | A member of the class of dithiocarbamic acids that is diethylcarbamic acid in which both of the oxygens are replaced by sulfur. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
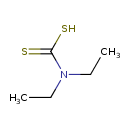
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N,N-Diethylcarbamodithioic acid | ChEBI | | N,N-Diethyldithiocarbamic acid | ChEBI | | N,N-Diethylcarbamodithioate | Generator | | N,N-Diethyldithiocarbamate | Generator | | Ammonium salt ditiocarb | MeSH | | Bismuth salt ditiocarb | MeSH | | Diethylcarbamodithioic acid | MeSH | | Diethyldithiocarbamate | MeSH | | Diethyldithiocarbamate, sodium | MeSH | | Diethyldithiocarbamate, zinc | MeSH | | Dithiocarb | MeSH | | Ditiocarb sodium | MeSH | | Ditiocarb, ammonium salt | MeSH | | Ditiocarb, bismuth salt | MeSH | | Ditiocarb, lead salt | MeSH | | Ditiocarb, potassium salt | MeSH | | Ditiocarb, sodium salt | MeSH | | Ditiocarb, sodium salt, trihydrate | MeSH | | Ditiocarb, tin(4+) salt | MeSH | | Ditiocarb, zinc salt | MeSH | | Imuthiol | MeSH | | Lead salt ditiocarb | MeSH | | Potassium salt ditiocarb | MeSH | | Sodium diethyldithiocarbamate | MeSH | | Sodium salt ditiocarb | MeSH | | Sodium, ditiocarb | MeSH | | Thiocarb | MeSH | | Zinc diethyldithiocarbamate | MeSH | | Zinc salt ditiocarb | MeSH | | Diethyldithiocarbamic acid | Generator | | Diethylcarbamodithioate | Generator | | Ditiocarb | MeSH | | Diethyl[sulphanyl(carbonothioyl)]amine | Generator |
|
|---|
| Chemical Formula | C5H11NS2 |
|---|
| Average Molecular Mass | 149.278 g/mol |
|---|
| Monoisotopic Mass | 149.033 g/mol |
|---|
| CAS Registry Number | 147-84-2 |
|---|
| IUPAC Name | diethyl[sulfanyl(carbonothioyl)]amine |
|---|
| Traditional Name | diethyldithiocarbamate |
|---|
| SMILES | CCN(CC)C(S)=S |
|---|
| InChI Identifier | InChI=1S/C5H11NS2/c1-3-6(4-2)5(7)8/h3-4H2,1-2H3,(H,7,8) |
|---|
| InChI Key | LMBWSYZSUOEYSN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as organosulfur compounds. These are organic compounds containing a carbon-sulfur bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organosulfur compounds |
|---|
| Class | Not Available |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Organosulfur compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organosulfur compound
- Organonitrogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-9300000000-7d8635ec0cb00b35f92a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-2900000000-a1cbfa24d8dba0a4285a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0g4i-7900000000-1d54ebcbd09eb9175de1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002f-9000000000-6b2a618d9e54c38c66e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-006t-6900000000-7c627e873b5f167bbc8e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9200000000-4ed977feecae07b36563 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-f9a8341fc944b4125ee9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-6c99eb8d1a3fda664321 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014r-5900000000-a22cdcf64fd39cbf306e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-9000000000-3be741399a9d72ed18b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03dr-8900000000-55aea3fa4bc3b15a69a1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9100000000-6dc62b5a0c9ccc1a48c7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-8137fa596dc94a01a293 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB02520 |
|---|
| HMDB ID | HMDB0251244 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8639 |
|---|
| ChEBI ID | 144353 |
|---|
| PubChem Compound ID | 8987 |
|---|
| Kegg Compound ID | C19150 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|