| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 11:42:05 UTC |
|---|
| Update Date | 2016-11-09 01:23:03 UTC |
|---|
| Accession Number | CHEM044102 |
|---|
| Identification |
|---|
| Common Name | 1,2-bis(2-ethylhexyl) 3,4,5,6-tetrachlorobenzene-1,2-dicarboxylate |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
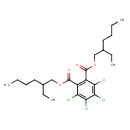
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,2-Bis(2-ethylhexyl) 3,4,5,6-tetrachlorobenzene-1,2-dicarboxylic acid | Generator |
|
|---|
| Chemical Formula | C24H34Cl4O4 |
|---|
| Average Molecular Mass | 528.330 g/mol |
|---|
| Monoisotopic Mass | 526.121 g/mol |
|---|
| CAS Registry Number | 34832-88-7 |
|---|
| IUPAC Name | 1,2-bis(2-ethylhexyl) 3,4,5,6-tetrachlorobenzene-1,2-dicarboxylate |
|---|
| Traditional Name | 1,2-bis(2-ethylhexyl) 3,4,5,6-tetrachlorophthalate |
|---|
| SMILES | CCCCC(CC)COC(=O)C1=C(C(=O)OCC(CC)CCCC)C(Cl)=C(Cl)C(Cl)=C1Cl |
|---|
| InChI Identifier | InChI=1S/C24H34Cl4O4/c1-5-9-11-15(7-3)13-31-23(29)17-18(20(26)22(28)21(27)19(17)25)24(30)32-14-16(8-4)12-10-6-2/h15-16H,5-14H2,1-4H3 |
|---|
| InChI Key | BELGUQVGZMFEAQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzoic acid esters. These are ester derivatives of benzoic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Benzoic acid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzoate ester
- Halobenzoic acid or derivatives
- 4-halobenzoic acid or derivatives
- 3-halobenzoic acid or derivatives
- 2-halobenzoic acid or derivatives
- Benzoyl
- Chlorobenzene
- Halobenzene
- Aryl chloride
- Aryl halide
- Dicarboxylic acid or derivatives
- Vinylogous halide
- Carboxylic acid ester
- Carboxylic acid derivative
- Organochloride
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Organohalogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01t9-0903080000-ef37fd51dae4a2d6431b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-6901010000-3c73c01269cbb2a7a1b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08fu-9410000000-7d84af0b231a2c3150a5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0102090000-cc381eb984864dabb047 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03fr-1526970000-49648371c2e21086bd9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9345100000-5d44dc2d360e0787f361 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 118190 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|