| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 11:29:24 UTC |
|---|
| Update Date | 2016-11-09 01:23:01 UTC |
|---|
| Accession Number | CHEM043963 |
|---|
| Identification |
|---|
| Common Name | lasofoxifene |
|---|
| Class | Small Molecule |
|---|
| Description | Lasofoxifene is a non-steroidal 3rd generation selective estrogen receptor modulator (SERM) that selectively binds to both ERα and ERβ with high affinity. It is a naphthalene derivative marketed for prevention and treatment of osteoporosis and for the treatment of vaginal atrophy. It was initially developed as Oporia by Pfizer as a treatment for postmenopausal osteoporosis and vaginal atrophy, in which were both rejected for approval by FDA. Later Fablyn was developed as a result of a research collaboration between Pfizer and Ligand Pharmaceuticals with a newly submitted New Drug Application in 2008. It gained approval by European Commission in March 2009. Ligand Pharmaceuticals signed a license agreement with Sermonix Pharmaceuticals for the development and commercialization of oral lasofoxifene in the USA. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
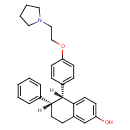
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Lasofoxifene hydrochloride | MeSH | | (-)-cis-5,6,7,8-tetrahydro-6-Phenyl-5-(P-(2-(1-pyrrolidinyl)ethoxy)phenyl)-2-naphthol | MeSH | | cis-1R-(4'-Pyrrolidinoethoxyphenyl)-2S-phenyl-6-hydroxy-1,2,3,4-tetrahydronaphthalene, tartrate salt | MeSH | | LAS estrogen receptor modulator | MeSH |
|
|---|
| Chemical Formula | C28H31NO2 |
|---|
| Average Molecular Mass | 413.551 g/mol |
|---|
| Monoisotopic Mass | 413.235 g/mol |
|---|
| CAS Registry Number | 180916-16-9 |
|---|
| IUPAC Name | (5R,6S)-6-phenyl-5-{4-[2-(pyrrolidin-1-yl)ethoxy]phenyl}-5,6,7,8-tetrahydronaphthalen-2-ol |
|---|
| Traditional Name | lasofoxifene |
|---|
| SMILES | [H][C@@]1(CCC2=CC(O)=CC=C2[C@@]1([H])C1=CC=C(OCCN2CCCC2)C=C1)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C28H31NO2/c30-24-11-15-27-23(20-24)10-14-26(21-6-2-1-3-7-21)28(27)22-8-12-25(13-9-22)31-19-18-29-16-4-5-17-29/h1-3,6-9,11-13,15,20,26,28,30H,4-5,10,14,16-19H2/t26-,28+/m1/s1 |
|---|
| InChI Key | GXESHMAMLJKROZ-IAPPQJPRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylnaphthalenes. Phenylnaphthalenes are compounds containing a phenylnaphthalene skeleton, which consists of a naphthalene bound to a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Naphthalenes |
|---|
| Sub Class | Phenylnaphthalenes |
|---|
| Direct Parent | Phenylnaphthalenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylnaphthalene
- Stilbene
- Tetralin
- Phenoxy compound
- Phenol ether
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- N-alkylpyrrolidine
- Pyrrolidine
- Tertiary aliphatic amine
- Tertiary amine
- Ether
- Azacycle
- Organoheterocyclic compound
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-2021900000-fa2afd3a45e77e79caff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9142100000-aa9a530d9148928aca60 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0592-9121000000-aa9348b7693d712742d9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-1002900000-e9d0c9bba98fa211160c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02t9-3029400000-c4994f82dc45badd2774 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01b9-8089000000-27271370f02794cae069 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB06202 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Lasofoxifene |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 216416 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|