| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 11:28:27 UTC |
|---|
| Update Date | 2016-11-09 01:23:01 UTC |
|---|
| Accession Number | CHEM043948 |
|---|
| Identification |
|---|
| Common Name | Quinaprilat |
|---|
| Class | Small Molecule |
|---|
| Description | A dicarboxylic acid resulting from the hydrolysis of the ethyl ester group of quinapril to give the corresponding dicarboxylic acid. The active angiotensin-converting enzyme inhibitor (ACE inhibitor) of the prodrug quinapril. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
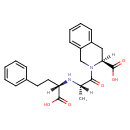
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| CI 928 | ChEBI | | CI-928 | ChEBI | | CI928 | ChEBI | | CL-928 | ChEBI | | Quinaprilate | ChEBI | | Quinaprilatum | ChEBI | | Quinaprilic acid | Generator | | CI-928quinaprilat | HMDB | | 2-(2-((1-Carboxy-3-phenylpropyl)amino)-1-oxopropyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic acid | HMDB | | Quinaprilat, (3S-(2(r*(r*)),3R*))-isomer | HMDB |
|
|---|
| Chemical Formula | C23H26N2O5 |
|---|
| Average Molecular Mass | 410.463 g/mol |
|---|
| Monoisotopic Mass | 410.184 g/mol |
|---|
| CAS Registry Number | 82768-85-2 |
|---|
| IUPAC Name | (3S)-2-[(2S)-2-{[(1S)-1-carboxy-3-phenylpropyl]amino}propanoyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid |
|---|
| Traditional Name | quinaprilat |
|---|
| SMILES | [H][C@@](C)(N[C@@]([H])(CCC1=CC=CC=C1)C(O)=O)C(=O)N1CC2=CC=CC=C2C[C@@]1([H])C(O)=O |
|---|
| InChI Identifier | InChI=1S/C23H26N2O5/c1-15(24-19(22(27)28)12-11-16-7-3-2-4-8-16)21(26)25-14-18-10-6-5-9-17(18)13-20(25)23(29)30/h2-10,15,19-20,24H,11-14H2,1H3,(H,27,28)(H,29,30)/t15-,19-,20-/m0/s1 |
|---|
| InChI Key | FLSLEGPOVLMJMN-YSSFQJQWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Dipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-dipeptide
- N-acyl-l-alpha-amino acid
- Alpha-amino acid amide
- Alpha-amino acid
- Alpha-amino acid or derivatives
- Tetrahydroisoquinoline
- L-alpha-amino acid
- Aralkylamine
- Monocyclic benzene moiety
- Dicarboxylic acid or derivatives
- Benzenoid
- Tertiary carboxylic acid amide
- Amino acid or derivatives
- Carboxamide group
- Amino acid
- Organoheterocyclic compound
- Azacycle
- Carboxylic acid
- Secondary aliphatic amine
- Secondary amine
- Organopnictogen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Amine
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0403-2925000000-e4de4c2b1f51a90ebc34 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-000i-9642440000-3935bd20baf3ed736d1d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03xu-0239400000-657f7ef4a13d754d6019 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1953000000-6560d0372a2a1dd70171 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03ec-2900000000-89636c81c5cbbdc3ef6d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0aor-0009500000-9c12c7c6c289ed26d187 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0690-0529100000-5ebce1d9aed5907f8abf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02e9-1910000000-df2dae9a4c8d21668f20 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0125900000-de537442df409f3872dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0319100000-0d99754c5f3bea7a6b30 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02u3-2900000000-67e4c42bed2130a5a52d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0114900000-c3d294563f563dcc6f6d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-057i-0911100000-7452d726559f5d57738a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0m0x-2910000000-ad031a0b0ef9b18dd397 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0042005 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Quinaprilat |
|---|
| Chemspider ID | 97106 |
|---|
| ChEBI ID | 140296 |
|---|
| PubChem Compound ID | 107994 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. https://www.ncbi.nlm.nih.gov/pubmed/?term=11300367 | | 2. https://www.ncbi.nlm.nih.gov/pubmed/?term=11422007 | | 3. https://www.ncbi.nlm.nih.gov/pubmed/?term=11824807 | | 4. https://www.ncbi.nlm.nih.gov/pubmed/?term=12698173 | | 5. https://www.ncbi.nlm.nih.gov/pubmed/?term=12808303 | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=15223904 | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=19016233 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=19135197 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=19761414 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=21083190 | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=24175935 | | 12. https://www.ncbi.nlm.nih.gov/pubmed/?term=9336392 | | 13. Kieback AG, Felix SB, Reffelmann T: Quinaprilat: a review of its pharmacokinetics, pharmacodynamics, toxicological data and clinical application. Expert Opin Drug Metab Toxicol. 2009 Oct;5(10):1337-47. doi: 10.1517/17425250903282773. | | 14. Steinhauff S, Pehlivanli S, Bakovic-Alt R, Meiser BM, Becker BF, von Scheidt W, Weis M: Beneficial effects of quinaprilat on coronary vasomotor function, endothelial oxidative stress, and endothelin activation after human heart transplantation. Transplantation. 2004 Jun 27;77(12):1859-65. doi: 10.1097/01.tp.0000131148.78203.b7. |
|
|---|