| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 11:27:43 UTC |
|---|
| Update Date | 2016-11-09 01:23:01 UTC |
|---|
| Accession Number | CHEM043938 |
|---|
| Identification |
|---|
| Common Name | Clostebol acetate |
|---|
| Class | Small Molecule |
|---|
| Description | Clostebol acetate (BAN) (brand names Macrobin, Steranabol, Alfa-Trofodermin, Megagrisevit), also known as 4-chlorotestosterone 17β-acetate (4-CLTA) or as 4-chloroandrost-4-en-17β-ol-3-one 17β-acetate, is a synthetic, injected anabolic-androgenic steroid (AAS) and a derivative of testosterone that is marketed in Germany and Italy. It is an androgen ester – specifically, the C17β acetate ester of clostebol (4-chlorotestosterone) – and acts as a prodrug of clostebol in the body. Clostebol acetate is administered via intramuscular injection. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
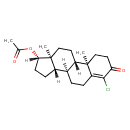
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Clostebol 17 beta-acetate | Kegg | | Trofodermin | Kegg | | Clostebol 17 b-acetate | Generator | | Clostebol 17 b-acetic acid | Generator | | Clostebol 17 beta-acetic acid | Generator | | Clostebol 17 β-acetate | Generator | | Clostebol 17 β-acetic acid | Generator | | Clostebol acetic acid | Generator | | Steranobol | MeSH | | 4-Chloroandrost-4-ene-17beta-ol-3-one acetate | MeSH | | 4-Chlorotestosterone acetate | MeSH | | Macrobin | MeSH | | Clostebolacetate | MeSH | | Turinabol | MeSH | | 4-Chlorotestosterone 17-acetate | MeSH | | Clostebol-17-acetate | MeSH | | 4-CLTA | MeSH |
|
|---|
| Chemical Formula | C21H29ClO3 |
|---|
| Average Molecular Mass | 364.910 g/mol |
|---|
| Monoisotopic Mass | 364.181 g/mol |
|---|
| CAS Registry Number | 855-19-6 |
|---|
| IUPAC Name | (1S,2R,10R,11S,14S,15S)-6-chloro-2,15-dimethyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-14-yl acetate |
|---|
| Traditional Name | (1S,2R,10R,11S,14S,15S)-6-chloro-2,15-dimethyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-14-yl acetate |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])CCC4=C(Cl)C(=O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)OC(C)=O |
|---|
| InChI Identifier | InChI=1S/C21H29ClO3/c1-12(23)25-18-7-6-14-13-4-5-16-19(22)17(24)9-11-20(16,2)15(13)8-10-21(14,18)3/h13-15,18H,4-11H2,1-3H3/t13-,14-,15-,18-,20+,21-/m0/s1 |
|---|
| InChI Key | XYGMEFJSKQEBTO-KUJXMBTLSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as steroid esters. Steroid esters are compounds containing a steroid moiety which bears a carboxylic acid ester group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroid esters |
|---|
| Direct Parent | Steroid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Steroid ester
- Androgen-skeleton
- Androstane-skeleton
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- 4-halo-steroid
- Oxosteroid
- Halo-steroid
- Delta-4-steroid
- Cyclohexenone
- Alpha-haloketone
- Alpha-chloroketone
- Carboxylic acid ester
- Cyclic ketone
- Ketone
- Chloroalkene
- Carboxylic acid derivative
- Haloalkene
- Vinyl halide
- Vinyl chloride
- Monocarboxylic acid or derivatives
- Organic oxide
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Organochloride
- Carbonyl group
- Organohalogen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01b9-0019000000-c2c9905fd510b6d83cfb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ab9-0269000000-507275205b148d4d5d59 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01p9-1291000000-f268303136805a789881 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03k9-0009000000-0e83ff1909149c06413f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0229-2009000000-3d5d00ed058f6c13a8f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052o-8096000000-fb7c1069554c02e70418 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Clostebol acetate |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 13327 |
|---|
| Kegg Compound ID | C18374 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|