| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 11:24:44 UTC |
|---|
| Update Date | 2016-11-09 01:23:00 UTC |
|---|
| Accession Number | CHEM043895 |
|---|
| Identification |
|---|
| Common Name | tilidin |
|---|
| Class | Small Molecule |
|---|
| Description | An ethyl 2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate that has S configuration at the carbon bearing the phenyl group and R configuration at the carbon bearing the dimethylamino group. The opioid analgesic tilidine is the racemate comprising equimolar amounts of dextilidine and its enantiomer, ent-dextilidine. A prodrug, tilidine is converted by the liver to the active analgesic, nortilidine; virtually all of the opioid activity resides in the (1S,2R) isomer (i.e. the isomer derived from dextilidine). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
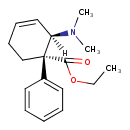
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-(1S,2R)-Ethyl-2-dimethylamino-1-phenyl-3-cyclohexen-1-carboxylate | ChEBI | | (+)-Ethyl trans-2-(dimethylamino)-1-phenyl-3-cyclohexene-1-carboxylate | ChEBI | | (1S,2R)-(+)-Ethyl-2-dimethylamino-1-phenyl-3-cyclohexen-1-carboxylate | ChEBI | | Dextilidina | ChEBI | | Dextilidinum | ChEBI | | (+)-(1S,2R)-Ethyl-2-dimethylamino-1-phenyl-3-cyclohexen-1-carboxylic acid | Generator | | (+)-Ethyl trans-2-(dimethylamino)-1-phenyl-3-cyclohexene-1-carboxylic acid | Generator | | (1S,2R)-(+)-Ethyl-2-dimethylamino-1-phenyl-3-cyclohexen-1-carboxylic acid | Generator | | Tilidine hydrochloride, (+-)-211trans | MeSH | | Tilidin godecke | MeSH | | Valerone | MeSH | | Godecke, tilidin | MeSH | | Hydrochloride, tilidine | MeSH | | Valoron | MeSH | | Tilidine hydrochloride | MeSH | | Godecke brand OF tilidine hydrochloride hemihydrate | MeSH | | Tilidate | MeSH | | Tilidine hydrochloride, (+)-trans | MeSH | | Tilidine | MeSH |
|
|---|
| Chemical Formula | C17H23NO2 |
|---|
| Average Molecular Mass | 273.376 g/mol |
|---|
| Monoisotopic Mass | 273.173 g/mol |
|---|
| CAS Registry Number | 20380-58-9 |
|---|
| IUPAC Name | ethyl (1S,2R)-2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate |
|---|
| Traditional Name | ethyl (1S,2R)-2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate |
|---|
| SMILES | [H][C@]1(C=CCC[C@]1(C(=O)OCC)C1=CC=CC=C1)N(C)C |
|---|
| InChI Identifier | InChI=1S/C17H23NO2/c1-4-20-16(19)17(14-10-6-5-7-11-14)13-9-8-12-15(17)18(2)3/h5-8,10-12,15H,4,9,13H2,1-3H3/t15-,17+/m1/s1 |
|---|
| InChI Key | WDEFBBTXULIOBB-WBVHZDCISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aralkylamines. These are alkylamines in which the alkyl group is substituted at one carbon atom by an aromatic hydrocarbyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Amines |
|---|
| Direct Parent | Aralkylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aralkylamine
- Monocyclic benzene moiety
- Benzenoid
- Amino acid or derivatives
- Carboxylic acid ester
- Tertiary amine
- Tertiary aliphatic amine
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Organopnictogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | - ethyl 2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate (CHEBI:77822 )
|
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-9320000000-82e133a0dbc5f6946c05 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0090000000-549281aa0b1155af0315 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0h6s-3590000000-007ff8e16a196484312f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k96-9520000000-cbc61d8b586aef0ec9e9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-2d94511a58089153f832 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0umi-1290000000-0a195aeae1a84976ef2b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ul0-3950000000-dada302b3cdf7206624c | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB13787 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Tilidine |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 77822 |
|---|
| PubChem Compound ID | 30131 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|