| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 11:22:39 UTC |
|---|
| Update Date | 2016-11-09 01:23:00 UTC |
|---|
| Accession Number | CHEM043865 |
|---|
| Identification |
|---|
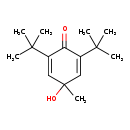
| Common Name | 2,6-Di-tert-butyl-4-hydroxy-4-methyl-2,5-cyclohexadien-1-one |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,6-Di-tert-butyl-4-hydroxy-4-methyl-2,5-cyclohexadien-1-one | MeSH | | 2,6-Bis(1,1-dimethylethyl)-4-hydroxy-4-methyl-2,5-cyclohexadien-1-one | HMDB | | 2,6-Di(tert-butyl)-4-hydroxy-4-methyl-2,5-cyclohexadien-1-one | HMDB | | 2,6-Di-tert-butyl-4-hydroxy-4-methyl-2,5-cyclohexadienone | HMDB | | 2,6-Di-tert-butyl-4-methyl-4-hydroxy-2,5-cyclohexadien-1-one | HMDB | | 2,6-Ditert-butyl-4-hydroxy-4-methylcyclohexa-2,5-dien-1-one | HMDB | | 4-Hydroxy-4-methyl-2,6-di-tert-butyl-2,5-cyclohexadienone | HMDB | | BHT-quinol | HMDB |
|
|---|
| Chemical Formula | C15H24O2 |
|---|
| Average Molecular Mass | 236.355 g/mol |
|---|
| Monoisotopic Mass | 236.178 g/mol |
|---|
| CAS Registry Number | 10396-80-2 |
|---|
| IUPAC Name | 2,6-di-tert-butyl-4-hydroxy-4-methylcyclohexa-2,5-dien-1-one |
|---|
| Traditional Name | 2,6-di-tert-butyl-4-hydroxy-4-methylcyclohexa-2,5-dien-1-one |
|---|
| SMILES | CC(C)(C)C1=CC(C)(O)C=C(C1=O)C(C)(C)C |
|---|
| InChI Identifier | InChI=1S/C15H24O2/c1-13(2,3)10-8-15(7,17)9-11(12(10)16)14(4,5)6/h8-9,17H,1-7H3 |
|---|
| InChI Key | DQBHJILNHNRDTM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as monocyclic monoterpenoids. These are monoterpenoids containing 1 ring in the isoprene chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Monocyclic monoterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monocyclic monoterpenoid
- Tertiary alcohol
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-02os-4940000000-74d1c19f1a4219cc3a55 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-0090000000-ded3d904ebb9d67ef04e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-02u9-2690000000-9a90dc3b253d4ea9380a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053r-9610000000-375c5f29d6f71a6b2453 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-c382ade86833a96572f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-17a554389c5b5527c9f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-07yr-3690000000-2df7a13aee3d603a41d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0190000000-7feaadbcc50ec3cbf0b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06si-9710000000-5709196b7a162be280fb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9400000000-ec314e2f09fa22bf80af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-c0031dc201eac6b6350a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-c0031dc201eac6b6350a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ik9-0790000000-dea4db79a24e1bd0484d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0166723 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 128880 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 146102 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|