| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 11:17:17 UTC |
|---|
| Update Date | 2016-11-09 01:22:59 UTC |
|---|
| Accession Number | CHEM043803 |
|---|
| Identification |
|---|
| Common Name | Fenproporex |
|---|
| Class | Small Molecule |
|---|
| Description | Fenproporex is an orally active stimulant drug, which was developed in the 1960s. It is used as an appetite suppressant and a treatment for obesity. It is listed as an illicit substance in many countries due to addiction issues and listed as a prohibited substance by the World Anti-Doping Agency. Structurally, fenproporex (N-2-cyanoethylamphetamine) falls within the phenylethamine and amphetamine chemical class of drugs. The N-2-cyanoethyl substituent was once believed to be resistant to cleavage, because fenproporex -- once recommended as an obesity treatment for patients with cardiovascular disease -- was originally claimed to lack stimulant properties. Contrary to the claim, research has demonstrated easy in vivo cleavage of the N-2-cyanothyl substituent to yield amphetamine as a metabolite. However, in clinical practice, central nervous system stimulative effects are less notorious than with some other agents such as diethylpropion and mazindol.
In the United States fenproporex was never approved by the FDA for clinical use due to a lack of efficacy and safety data, and is listed as a drug in Schedule IV of the Controlled Substances Act. In 2006 and 2009, the FDA issued warnings that it had been detected in diet pills sold online, and imported from foreign manufacturers.
Despite being banned in the United States, fenproporex has been described as the second most commonly consumed appetite suppressant worldwide, with fenproporex containing anorectics still being commonly prescribed in South America. Little is known about the specific hazards of amphetamine based diet pills, however case reports have noted side effects such as chest pain, palpitations, headaches, and insomnia. In addition, placebo controlled studies have shown that participants using fenproporex experience more joint pain, sweating, blurred vision and tremor. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
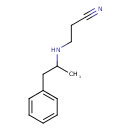
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Fenorex | Kegg | | Fenproporex monohydrochloride | MeSH | | Fenproporex monohydrochloride, (+-)-isomer | MeSH | | Ifa diety | MeSH | | Fenproporex, (+-)-isomer | MeSH |
|
|---|
| Chemical Formula | C12H16N2 |
|---|
| Average Molecular Mass | 188.269 g/mol |
|---|
| Monoisotopic Mass | 188.131 g/mol |
|---|
| CAS Registry Number | 16397-28-7 |
|---|
| IUPAC Name | 3-[(1-phenylpropan-2-yl)amino]propanenitrile |
|---|
| Traditional Name | fenproporex |
|---|
| SMILES | CC(CC1=CC=CC=C1)NCCC#N |
|---|
| InChI Identifier | InChI=1S/C12H16N2/c1-11(14-9-5-8-13)10-12-6-3-2-4-7-12/h2-4,6-7,11,14H,5,9-10H2,1H3 |
|---|
| InChI Key | IQUFSXIQAFPIMR-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as amphetamines and derivatives. These are organic compounds containing or derived from 1-phenylpropan-2-amine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenethylamines |
|---|
| Direct Parent | Amphetamines and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Amphetamine or derivatives
- Phenylpropane
- Aralkylamine
- Secondary amine
- Nitrile
- Carbonitrile
- Secondary aliphatic amine
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014m-9600000000-4638d290c61f959a86b9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1900000000-39ea645836ae34df3bad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0v4i-8900000000-fc50b0ee9f48878b7629 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udl-9200000000-2a058391d3ae665a7bbc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-5dae440ca644f76a25b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-2900000000-dd0429db7b0e0780f65d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9500000000-4e2cd93f29835fd4b069 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-3900000000-d798f55f0f3645c7a53d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9100000000-ea7ddc7550ea36a99285 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-f14fb4de4c22179b8690 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-3900000000-3fdf6204e62b10634055 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9700000000-a45303877a0eafe2ea1b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-5cd14ec8651fea008118 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01550 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Fenproporex |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 61810 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|