| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 11:12:29 UTC |
|---|
| Update Date | 2016-11-09 01:22:58 UTC |
|---|
| Accession Number | CHEM043727 |
|---|
| Identification |
|---|
| Common Name | 2,5-dimethoxy-4-methylamphetamine |
|---|
| Class | Small Molecule |
|---|
| Description | A psychedelic phenyl isopropylamine derivative, commonly called DOM, whose mood-altering effects and mechanism of action may be similar to those of LSD. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
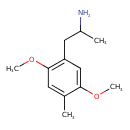
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(25-Dimethoxy-4-methyl-phenyl)-1-methyl-ethylamine | HMDB | | DOM | HMDB | | 2 5 DOM | HMDB | | 2,5 Dimethoxy 4 methylamphetamine | HMDB | | 2,5-Dimethoxy-4-methylamphetamine, (S)-isomer | HMDB | | 2,5-Dimethoxy-4-methylamphetamine hydrochloride, (R)-isomer | HMDB | | 2,5-Dimethoxy-4-methylamphetamine, hydrochloride | HMDB | | 2,5-Dimethoxy-4-methylamphetamine hydrochloride, (S)-isomer | HMDB | | 2,5-Dimethoxy-4-methylamphetamine, (+,-)-isomer | HMDB | | 2,5-Dimethoxy-4-methylamphetamine, (R)-isomer | HMDB | | 1-(2,5-Dimethoxy-4-methylphenyl)-2-aminopropane | HMDB | | 2,5-Dimethoxy-4-methylamphetamine hydrochloride, (+,-)-isomer | HMDB | | 2,5-Dimethoxy-4-methylamphetamine | MeSH |
|
|---|
| Chemical Formula | C12H19NO2 |
|---|
| Average Molecular Mass | 209.285 g/mol |
|---|
| Monoisotopic Mass | 209.142 g/mol |
|---|
| CAS Registry Number | 15588-95-1 |
|---|
| IUPAC Name | 1-(2,5-dimethoxy-4-methylphenyl)propan-2-amine |
|---|
| Traditional Name | (RS)-dom |
|---|
| SMILES | COC1=CC(CC(C)N)=C(OC)C=C1C |
|---|
| InChI Identifier | InChI=1S/C12H19NO2/c1-8-5-12(15-4)10(6-9(2)13)7-11(8)14-3/h5,7,9H,6,13H2,1-4H3 |
|---|
| InChI Key | NTJQREUGJKIARY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as amphetamines and derivatives. These are organic compounds containing or derived from 1-phenylpropan-2-amine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenethylamines |
|---|
| Direct Parent | Amphetamines and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Amphetamine or derivatives
- P-dimethoxybenzene
- Dimethoxybenzene
- Phenylpropane
- Phenoxy compound
- Anisole
- Phenol ether
- Methoxybenzene
- Alkyl aryl ether
- Aralkylamine
- Toluene
- Ether
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Amine
- Primary amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0006-9100000000-466e6b5613c401a0fa96 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-6900000000-776d7576e097d8efa08b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dl-0980000000-d874c558243b20796dde | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ox-2930000000-2c52c4614bb120efb5ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ik9-4900000000-1fa2fbdf5cdc97ac738d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0290000000-fe950e9a3fb343078146 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4l-0960000000-433bb60470f3961f122c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03fu-2900000000-083d504731cf5c946a4d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01ox-0940000000-676d5eac9118e13eab4c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03xu-0940000000-db8f6661c8e1f052c18c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05o0-4910000000-f4d9ed4eccc36ec1adb3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0190000000-2d34296744121f27404d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a59-0950000000-e64fef2389f47938c739 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-0960000000-68fe3fb78846a938d526 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01528 |
|---|
| HMDB ID | HMDB0245501 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | 2,5-Dimethoxy-4-methylamphetamine |
|---|
| Chemspider ID | 77462 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 85875 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|