| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 10:27:31 UTC |
|---|
| Update Date | 2016-11-09 01:22:49 UTC |
|---|
| Accession Number | CHEM042955 |
|---|
| Identification |
|---|
| Common Name | mannomustine |
|---|
| Class | Small Molecule |
|---|
| Description | Mannomustine (INN), also known as mannitol nitrogen mustard, tradename Degranol is an old alkylating antineoplastic agent from the group of nitrogen mustards. It was first synthesized and characterized in 1957 by Vargha et al.The mechanism of antineoplastic activity of mannomustine, like for all other alkylating agents, lies in its ability to alkylate DNA guanine nucleobases and, thus, to prevent uncoupling of DNA strands, which is a required step for any cell to divide.
Mannomustine was, at the time of its creation as a drug, claimed to be considerably less toxic than mechlorethamine. For example, the LD50 in rats, for intravenous mannomustine administration route, is claimed to be about 56 mg/kg. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
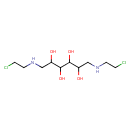
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Degranol | MeSH | | Mustard, mannitol | MeSH | | Mustard, mannitol nitrogen | MeSH | | Mannitol mustard | MeSH | | Decranol | MeSH | | Mannitlost | MeSH | | Nitrogen mustard, mannitol | MeSH | | Mannitol nitrogen mustard | MeSH |
|
|---|
| Chemical Formula | C10H22Cl2N2O4 |
|---|
| Average Molecular Mass | 305.200 g/mol |
|---|
| Monoisotopic Mass | 304.096 g/mol |
|---|
| CAS Registry Number | 576-68-1 |
|---|
| IUPAC Name | (2-chloroethyl)({6-[(2-chloroethyl)amino]-2,3,4,5-tetrahydroxyhexyl})amine |
|---|
| Traditional Name | mannomustine |
|---|
| SMILES | OC(CNCCCl)C(O)C(O)C(O)CNCCCl |
|---|
| InChI Identifier | InChI=1S/C10H22Cl2N2O4/c11-1-3-13-5-7(15)9(17)10(18)8(16)6-14-4-2-12/h7-10,13-18H,1-6H2 |
|---|
| InChI Key | MQXVYODZCMMZEM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,3-aminoalcohols. These are organic compounds containing an alkyl chain with an amine group bound to the C1 atom and an alcohol group bound to the C3 atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Amines |
|---|
| Direct Parent | 1,3-aminoalcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,3-aminoalcohol
- 1,2-aminoalcohol
- Secondary alcohol
- Secondary aliphatic amine
- Secondary amine
- Polyol
- Organic oxygen compound
- Organooxygen compound
- Organochloride
- Organohalogen compound
- Hydrocarbon derivative
- Alkyl halide
- Alkyl chloride
- Alcohol
- Organopnictogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-2139000000-5117a2a266d437901a8d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052f-9552000000-b9bb5129913609f54771 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-9000000000-d771bca2e4a67a581fbd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-1915000000-dbc1005cee9fda9d81ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-4910000000-2551fc1c4c2e56748f83 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004l-9200000000-f6134ebcec273eff1732 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Mannomustine |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 11337 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|