| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 10:26:57 UTC |
|---|
| Update Date | 2016-11-09 01:22:49 UTC |
|---|
| Accession Number | CHEM042942 |
|---|
| Identification |
|---|
| Common Name | homofenazine |
|---|
| Class | Small Molecule |
|---|
| Description | A phenothiazine derivative having a trifluoromethyl subsitituent at the 2-position and a 3-propyl group at the N-10 position. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Hexahydro-4-(3-(2-(trifluoromethyl)phenothiazin-10-yl)propyl)-1H-1,4-diazepin-1-ethanol | ChEBI | | Hexahydro-4-(3-(2-(trifluoromethyl)phenothiazin-10-yl)propyl)-1H-1,4-diazepine-1-ethanol | ChEBI | | Homofenazina | ChEBI | | Homofenazinum | ChEBI |
|
|---|
| Chemical Formula | C23H28F3N3OS |
|---|
| Average Molecular Mass | 451.550 g/mol |
|---|
| Monoisotopic Mass | 451.191 g/mol |
|---|
| CAS Registry Number | 3833-99-6 |
|---|
| IUPAC Name | 2-(4-{3-[2-(trifluoromethyl)-10H-phenothiazin-10-yl]propyl}-1,4-diazepan-1-yl)ethan-1-ol |
|---|
| Traditional Name | homofenazine |
|---|
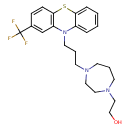
| SMILES | OCCN1CCCN(CCCN2C3=CC=CC=C3SC3=C2C=C(C=C3)C(F)(F)F)CC1 |
|---|
| InChI Identifier | InChI=1S/C23H28F3N3OS/c24-23(25,26)18-7-8-22-20(17-18)29(19-5-1-2-6-21(19)31-22)12-4-11-27-9-3-10-28(14-13-27)15-16-30/h1-2,5-8,17,30H,3-4,9-16H2 |
|---|
| InChI Key | LOHNHQLZFYCAEQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenothiazines. These are polycyclic aromatic compounds containing a phenothiazine moiety, which is a linear tricyclic system that consists of a two benzene rings joined by a para-thiazine ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzothiazines |
|---|
| Sub Class | Phenothiazines |
|---|
| Direct Parent | Phenothiazines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenothiazine
- Alkyldiarylamine
- Diarylthioether
- Aryl thioether
- Tertiary aliphatic/aromatic amine
- 1,4-diazepane
- Diazepane
- Para-thiazine
- Benzenoid
- 1,2-aminoalcohol
- Tertiary aliphatic amine
- Tertiary amine
- Azacycle
- Thioether
- Alkanolamine
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organofluoride
- Organohalogen compound
- Organic nitrogen compound
- Amine
- Alkyl halide
- Alkyl fluoride
- Primary alcohol
- Alcohol
- Hydrocarbon derivative
- Organopnictogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000900000-b4424bfed988fb3d4a15 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f8i-2622900000-cc283c71c3c297ff9437 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9634000000-08ab47e8327a0dec6e85 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000900000-1838221cf38e342a610d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0gi0-0173900000-37dc5e586a3ffdc0bf68 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-4191000000-dcee790ec2fd3b037540 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 59071 |
|---|
| PubChem Compound ID | 19687 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|