| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 10:21:53 UTC |
|---|
| Update Date | 2016-11-09 01:22:49 UTC |
|---|
| Accession Number | CHEM042878 |
|---|
| Identification |
|---|
| Common Name | sulfametrole |
|---|
| Class | Small Molecule |
|---|
| Description | A sulfonamide obtained by formal condensation of the sulfo group of 4-aminobenzenesulfonic acid with the amino group of 4-methoxy-1,2,5-thiadiazol-3-amine. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
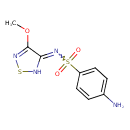
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N1-(4-Methoxy-1,2,5-thiadiazol-3-yl)sulfanilamide | ChEBI | | Sulfametrol | ChEBI | | Sulfametrolum | ChEBI | | Lidaprim | Kegg | | N1-(4-Methoxy-1,2,5-thiadiazol-3-yl)sulphanilamide | Generator | | Sulphametrol | Generator | | Sulphametrolum | Generator | | Sulphametrole | Generator | | N-(4-Methoxy-1,2,5-thiadiazol-3-yl)sulfanilamide | MeSH |
|
|---|
| Chemical Formula | C9H10N4O3S2 |
|---|
| Average Molecular Mass | 286.320 g/mol |
|---|
| Monoisotopic Mass | 286.019 g/mol |
|---|
| CAS Registry Number | 32909-92-5 |
|---|
| IUPAC Name | 4-amino-N-(4-methoxy-2,3-dihydro-1,2,5-thiadiazol-3-ylidene)benzene-1-sulfonamide |
|---|
| Traditional Name | 4-amino-N-(4-methoxy-2H-1,2,5-thiadiazol-3-ylidene)benzenesulfonamide |
|---|
| SMILES | COC1=NSNC1=NS(=O)(=O)C1=CC=C(N)C=C1 |
|---|
| InChI Identifier | InChI=1S/C9H10N4O3S2/c1-16-9-8(11-17-12-9)13-18(14,15)7-4-2-6(10)3-5-7/h2-5H,10H2,1H3,(H,11,13) |
|---|
| InChI Key | IZOYMGQQVNAMHS-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aminobenzenesulfonamides. These are organic compounds containing a benzenesulfonamide moiety with an amine group attached to the benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzenesulfonamides |
|---|
| Direct Parent | Aminobenzenesulfonamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aminobenzenesulfonamide
- Benzenesulfonyl group
- Aniline or substituted anilines
- Alkyl aryl ether
- Organosulfonic acid amide
- Imidolactam
- Azole
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Heteroaromatic compound
- Sulfonyl
- Aminosulfonyl compound
- Thiadiazole
- Ether
- Azacycle
- Organoheterocyclic compound
- Organic oxide
- Primary amine
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Amine
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-053r-8920000000-1efad122f050518cee2d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05g0-0590000000-6dc2575d4ab0f8009e91 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bt9-3930000000-22d5f0bfa713a89a8ec3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00b9-9300000000-6a55d8ab6448a84ad016 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-f5ecf972185013bf4b98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052r-1960000000-91d7c2b742e22d8b054e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9820000000-3e65480314d055457119 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0258577 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Sulfametrole |
|---|
| Chemspider ID | 58466 |
|---|
| ChEBI ID | 88258 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|