| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 10:09:32 UTC |
|---|
| Update Date | 2016-11-09 01:22:47 UTC |
|---|
| Accession Number | CHEM042752 |
|---|
| Identification |
|---|
| Common Name | ammonium 1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-henicosafluorodecane-1-sulfonate |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
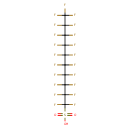
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Perfluorodecane sulfonate | Generator | | Perfluorodecane sulphonate | Generator | | Perfluorodecane sulphonic acid | Generator |
|
|---|
| Chemical Formula | C10HF21O3S |
|---|
| Average Molecular Mass | 600.140 g/mol |
|---|
| Monoisotopic Mass | 599.931 g/mol |
|---|
| CAS Registry Number | 67906-42-7 |
|---|
| IUPAC Name | henicosafluorodecane-1-sulfonic acid |
|---|
| Traditional Name | henicosafluorodecane-1-sulfonic acid |
|---|
| SMILES | OS(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |
|---|
| InChI Identifier | InChI=1S/C10HF21O3S/c11-1(12,3(15,16)5(19,20)7(23,24)9(27,28)29)2(13,14)4(17,18)6(21,22)8(25,26)10(30,31)35(32,33)34/h(H,32,33,34) |
|---|
| InChI Key | HYWZIAVPBSTISZ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as perfluoroalkyl sulfonic acid and derivatives. These are organic compounds containing an alkyl chain attached to the sulfur of a sulfonic acid group (or a derivative thereof), where all hydrogens of the alkyl chain are replaced by fluorine atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organohalogen compounds |
|---|
| Class | Alkyl halides |
|---|
| Sub Class | Alkyl fluorides |
|---|
| Direct Parent | Perfluoroalkyl sulfonic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Perfluoroalkyl sulfonic acid or derivatives
- Alkanesulfonic acid
- Sulfonyl
- Organosulfonic acid
- Organosulfonic acid or derivatives
- Organic sulfonic acid or derivatives
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organosulfur compound
- Organofluoride
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 50V, Negative | splash10-0002-0000090000-45d021ea3fdc9c1c95d8 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-0002-0000090000-5374258d8ec4374e4fcc | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-0002-0000090000-4830b58cd395a8cbc8e3 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000090000-87d2353ce5b03ceda179 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0000090000-2056bb2e8ce0567364c6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00lr-0811094000-c99bd2eb1448350a202e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fsi-0000093000-587903915002bec2cde9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gb9-0910000000-4015061601666bc2253c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0697000000-e4043212fc5d73d61bc3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kb-7000090000-54fabe581a10ae73930f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0729800000-6d1ec9c620cae9d6cba7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000009000-4e8cf16e0e1c04731927 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0000009000-4e8cf16e0e1c04731927 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fsi-1200392000-27818b96426691e570db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000090000-1767bb6d43a106cdc318 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0000090000-1767bb6d43a106cdc318 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-0000090000-1767bb6d43a106cdc318 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0246166 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 60955 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 67636 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|