| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 10:04:03 UTC |
|---|
| Update Date | 2016-11-09 01:22:45 UTC |
|---|
| Accession Number | CHEM042664 |
|---|
| Identification |
|---|
| Common Name | verteporfin (product), verteporfin (product), verteporfin (substance), verteporfin (substance), bpd-MA, bpd-MA, novartis brand of verteporfin, novartis brand of verteporfin |
|---|
| Class | Small Molecule |
|---|
| Description | Verteporfin, marketed as Visudyne, is a benzoporphyrin derivative. It is used as a photosensitizer in photodynamic therapy to eliminate abnormal blood vessels in wet form macular degeneration. Verteporfin accumulates in these abnormal blood vessels and, when stimulated by nonthermal red light with a wavelength of 693 nm in the presence of oxygen, produces highly reactive short-lived singlet oxygen and other reactive oxygen radicals, resulting in local damage to the endothelium and blockage of the vessels. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
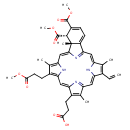
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-[(23S,24R)-14-Ethenyl-5-(3-methoxy-3-oxopropyl)-22,23-bis(methoxycarbonyl)-4,10,15,24-tetramethyl-25,26,27,28-tetraazahexacyclo[16.6.1.1³,⁶.1⁸,¹¹.1¹³,¹⁶.0¹⁹,²⁴]octacosa-1,3,5,7,9,11(27),12,14,16,18(25),19,21-dodecaen-9-yl]propanoate | HMDB |
|
|---|
| Chemical Formula | C41H42N4O8 |
|---|
| Average Molecular Mass | 718.794 g/mol |
|---|
| Monoisotopic Mass | 718.300 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 3-[(23S,24R)-14-ethenyl-5-(3-methoxy-3-oxopropyl)-22,23-bis(methoxycarbonyl)-4,10,15,24-tetramethyl-25,26,27,28-tetraazahexacyclo[16.6.1.1³,⁶.1⁸,¹¹.1¹³,¹⁶.0¹⁹,²⁴]octacosa-1,3,5,7,9,11(27),12,14,16,18(25),19,21-dodecaen-9-yl]propanoic acid |
|---|
| Traditional Name | VERT |
|---|
| SMILES | COC(=O)CCC1=C2NC(\C=C3/N=C(/C=C4\N\C(=C/C5=N/C(=C\2)/C(CCC(O)=O)=C5C)C(C=C)=C4C)C2=CC=C([C@@H](C(=O)OC)[C@@]32C)C(=O)OC)=C1C |
|---|
| InChI Identifier | InChI=1S/C41H42N4O8/c1-9-23-20(2)29-17-34-27-13-10-26(39(49)52-7)38(40(50)53-8)41(27,5)35(45-34)19-30-22(4)25(12-15-37(48)51-6)33(44-30)18-32-24(11-14-36(46)47)21(3)28(43-32)16-31(23)42-29/h9-10,13,16-19,38,42,44H,1,11-12,14-15H2,2-8H3,(H,46,47)/b28-16-,29-17-,30-19-,31-16-,32-18-,33-18-,34-17-,35-19-/t38-,41+/m0/s1 |
|---|
| InChI Key | YTZALCGQUPRCGW-MXVXOLGGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as chlorins. These are large heterocyclic aromatic ring systems consisting, at the core, of three pyrroles and one pyrroline coupled through four methine linkages. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Chlorins |
|---|
| Direct Parent | Chlorins |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uy0-0000009400-1d7c8f1fc2dc50cfe52f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0m0m-0000029100-7684d9a7f67a3f18d670 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-1000095000-628656af2025a7bd099e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014r-0000009600-89a0501bc164118f1867 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0aor-1000009100-26eeadf6aaa77d903859 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-056u-4000009000-eff443ca0bb459d0673d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00p0-0000009200-0cb74b4edd542c40054f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00mo-0000009100-b6fc818410d9eab222c4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03xr-0000029100-6af80c39f2d17512bce7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fr6-0000009400-844d3115b3be7e5767a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0597-0000029100-48abe90a5dc5b255b75a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03xs-1000059200-cf96237b152a02e2917c | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00460 |
|---|
| HMDB ID | HMDB0014603 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Verteporfin |
|---|
| Chemspider ID | 4515032 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Nowak-Sliwinska P, Karocki A, Elas M, Pawlak A, Stochel G, Urbanska K: Verteporfin, photofrin II, and merocyanine 540 as PDT photosensitizers against melanoma cells. Biochem Biophys Res Commun. 2006 Oct 20;349(2):549-55. Epub 2006 Aug 22. | | 2. Chan WM, Lim TH, Pece A, Silva R, Yoshimura N: Verteporfin PDT for non-standard indications--a review of current literature. Graefes Arch Clin Exp Ophthalmol. 2010 May;248(5):613-26. doi: 10.1007/s00417-010-1307-z. Epub 2010 Feb 17. |
|
|---|