| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 02:04:23 UTC |
|---|
| Update Date | 2016-11-09 01:22:41 UTC |
|---|
| Accession Number | CHEM042489 |
|---|
| Identification |
|---|
| Common Name | Protocatechuic acid 4-O-sulphate |
|---|
| Class | Small Molecule |
|---|
| Description | Protocatechuic acid 4-o-sulphate, also known as 3-hsob, is a member of the class of compounds known as phenylsulfates. Phenylsulfates are compounds containing a sulfuric acid group conjugated to a phenyl group. Protocatechuic acid 4-o-sulphate is slightly soluble (in water) and an extremely strong acidic compound (based on its pKa). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
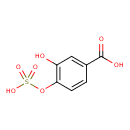
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Protocatechuate 4-O-sulfate | Generator | | Protocatechuate 4-O-sulphate | Generator | | Protocatechuic acid 4-O-sulfuric acid | Generator | | Protocatechuic acid 4-O-sulphuric acid | Generator | | 3-Hydroxy-sulfonyloxybenzoic acid | HMDB | | 3-HSOB | HMDB | | 3-Hydroxy-4-(sulfooxy)benzoic acid | HMDB | | Protocatechuic acid sulfate | HMDB | | Protocatechuic acid sulphate | HMDB | | 3,4-DHBA sulfate | HMDB | | 3,4-DHBA sulphate | HMDB |
|
|---|
| Chemical Formula | C7H6O7S |
|---|
| Average Molecular Mass | 234.180 g/mol |
|---|
| Monoisotopic Mass | 233.983 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 3-hydroxy-4-(sulfooxy)benzoic acid |
|---|
| Traditional Name | 3-hydroxy-4-(sulfooxy)benzoic acid |
|---|
| SMILES | OC(=O)C1=CC(O)=C(OS(O)(=O)=O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C7H6O7S/c8-5-3-4(7(9)10)1-2-6(5)14-15(11,12)13/h1-3,8H,(H,9,10)(H,11,12,13) |
|---|
| InChI Key | NGQYUDIZIPNWJV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylsulfates. Phenylsulfates are compounds containing a sulfuric acid group conjugated to a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic sulfuric acids and derivatives |
|---|
| Sub Class | Arylsulfates |
|---|
| Direct Parent | Phenylsulfates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylsulfate
- Hydroxybenzoic acid
- Benzoic acid or derivatives
- Benzoic acid
- Phenoxy compound
- Benzoyl
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Sulfuric acid ester
- Sulfate-ester
- Sulfuric acid monoester
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0uyi-1930000000-deb2d79f265b4d78fef5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0390000000-ccabed7b27f365f5acce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a5i-0910000000-db7894fff6e16da697fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-9600000000-eca3a36d6ed818fe4678 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0490000000-d91ee7d59a18f565a1cc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pc9-0910000000-52f458406ef93824b774 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-2900000000-cd6ebf2a6acafa9bc032 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0159-0090000000-70fd210f71ae39326901 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ap0-0930000000-21b7bd3deabbd872dc8d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-059j-8900000000-bfc1e338c96f342c2042 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-64544088b0077a7c18b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0930000000-6970a84c2f09d44c272d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000w-6900000000-bb35c50253bc33d60b5d | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0240382 |
|---|
| FooDB ID | FDB031321 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 169832 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|