| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 02:04:17 UTC |
|---|
| Update Date | 2016-11-09 01:22:41 UTC |
|---|
| Accession Number | CHEM042485 |
|---|
| Identification |
|---|
| Common Name | 4-Methyl-catechol-1-O-sulphate |
|---|
| Class | Small Molecule |
|---|
| Description | 4-methyl-catechol-1-o-sulphate is a member of the class of compounds known as phenylsulfates. Phenylsulfates are compounds containing a sulfuric acid group conjugated to a phenyl group. 4-methyl-catechol-1-o-sulphate is slightly soluble (in water) and an extremely strong acidic compound (based on its pKa). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
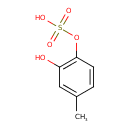
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-Methylcatechol 1-sulfuric acid | Generator | | 4-Methylcatechol 1-sulphate | Generator | | 4-Methylcatechol 1-sulphuric acid | Generator | | 4-Methyl-catechol-1-O-sulfate | HMDB | | 4-Methyl-catechol-1-O-sulfuric acid | HMDB | | 4-Methyl-catechol-1-O-sulphuric acid | HMDB | | 4-Methylcatechol monosulfate | HMDB | | 4-Methylcatechol monosulphate | HMDB | | 4-Methylcatechol sulfate | HMDB | | 4-Methylcatechol sulphate | HMDB | | 4-Methylcatechol 1-sulfate | HMDB |
|
|---|
| Chemical Formula | C7H8O5S |
|---|
| Average Molecular Mass | 204.200 g/mol |
|---|
| Monoisotopic Mass | 204.009 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2-hydroxy-4-methylphenyl)oxidanesulfonic acid |
|---|
| Traditional Name | (2-hydroxy-4-methylphenyl)oxidanesulfonic acid |
|---|
| SMILES | CC1=CC(O)=C(OS(O)(=O)=O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C7H8O5S/c1-5-2-3-7(6(8)4-5)12-13(9,10)11/h2-4,8H,1H3,(H,9,10,11) |
|---|
| InChI Key | NCOMUGOPQPRVSE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylsulfates. Phenylsulfates are compounds containing a sulfuric acid group conjugated to a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic sulfuric acids and derivatives |
|---|
| Sub Class | Arylsulfates |
|---|
| Direct Parent | Phenylsulfates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylsulfate
- Phenoxy compound
- M-cresol
- 1-hydroxy-4-unsubstituted benzenoid
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- Toluene
- Sulfuric acid ester
- Sulfate-ester
- Sulfuric acid monoester
- Monocyclic benzene moiety
- Benzenoid
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0290000000-5b3a7bd5cb6f719c1baf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a70-1920000000-d99eec971cd5630f10ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gb9-9000000000-8a717dc442de57f503e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-129f6b9cab97e786b90c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0930000000-87a2bc800110b7c6775c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05gi-7900000000-420b5f1c79308691c53a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-ca2df71642be8b8709e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-48d779cccda8483ad1a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0089-9300000000-ade25764440d58e79068 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-0920000000-25e47a18e27a087c0883 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0900000000-0fe17abac0494b705106 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002f-9100000000-5435e8da0d72fb337089 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0240459 |
|---|
| FooDB ID | FDB031316 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 59696770 |
|---|
| ChEBI ID | 176442 |
|---|
| PubChem Compound ID | 54104170 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. |
|
|---|