| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 02:04:05 UTC |
|---|
| Update Date | 2016-11-09 01:22:41 UTC |
|---|
| Accession Number | CHEM042476 |
|---|
| Identification |
|---|
| Common Name | γ-tocopherol |
|---|
| Class | Small Molecule |
|---|
| Description | γ-tocopherol is practically insoluble (in water) and a very weakly acidic compound (based on its pKa). γ-tocopherol can be found in a number of food items such as peanut, burbot, olive, and guava, which makes γ-tocopherol a potential biomarker for the consumption of these food products. Tocopherols (TCP) are a class of organic chemical compounds (more precisely, various methylated phenols), many of which have vitamin E activity. Because the vitamin activity was first identified in 1936 from a dietary fertility factor in rats, it was given the name "tocopherol" from the Greek words "τόκος" [tókos, birth], and "φέρειν", [phérein, to bear or carry] meaning in sum "to carry a pregnancy," with the ending "-ol" signifying its status as a chemical alcohol . |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
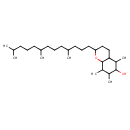
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C28H54O2 |
|---|
| Average Molecular Mass | 422.738 g/mol |
|---|
| Monoisotopic Mass | 422.412 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 5,7,8-trimethyl-2-(4,8,12-trimethyltridecyl)-octahydro-2H-1-benzopyran-6-ol |
|---|
| Traditional Name | 5,7,8-trimethyl-2-(4,8,12-trimethyltridecyl)-octahydro-2H-1-benzopyran-6-ol |
|---|
| SMILES | CC(C)CCCC(C)CCCC(C)CCCC1CCC2C(C)C(O)C(C)C(C)C2O1 |
|---|
| InChI Identifier | InChI=1S/C28H54O2/c1-19(2)11-8-12-20(3)13-9-14-21(4)15-10-16-25-17-18-26-24(7)27(29)22(5)23(6)28(26)30-25/h19-29H,8-18H2,1-7H3 |
|---|
| InChI Key | WIGCFUFOHFEKBI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Farsesane sesquiterpenoid
- Sesquiterpenoid
- Benzopyran
- Oxane
- Cyclic alcohol
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Ether
- Dialkyl ether
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05fr-1400900000-b40e1a0c2bf71485fb83 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fk9-2920100000-2ca4c9707e9c17820801 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9611000000-51254c1194735f45e81b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0110900000-92b923b6a3ac21515c92 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0g4i-0930800000-876cd90cbceeda457e64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-2951100000-64ccadc666897eaf9f3d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | FDB031304 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Tocopherol |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|