| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 02:02:59 UTC |
|---|
| Update Date | 2016-11-09 01:22:40 UTC |
|---|
| Accession Number | CHEM042443 |
|---|
| Identification |
|---|
| Common Name | ubiquinone-8 |
|---|
| Class | Small Molecule |
|---|
| Description | A ubiquinone whose structure comprises a 2,3-dimethoxy-5-methylbenzoquinone nucleus (common to all ubiquinones) and a side chain of eight isoprenoid units. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
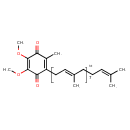
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Coenzyme Q8 | ChEBI | | Coenzyme-Q8 | ChEBI | | COQ8 | ChEBI | | Ubiquinone 8 | ChEBI | | Ubiquinone(8) | ChEBI | | Ubiquinone 40 | MeSH | | Ubiquinone Q8 | MeSH |
|
|---|
| Chemical Formula | C49H74O4 |
|---|
| Average Molecular Mass | 727.110 g/mol |
|---|
| Monoisotopic Mass | 726.559 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 2,3-dimethoxy-5-methyl-6-[(2E,6E,10E,14E,18E,22E,26E)-3,7,11,15,19,23,27,31-octamethyldotriaconta-2,6,10,14,18,22,26,30-octaen-1-yl]cyclohexa-2,5-diene-1,4-dione |
|---|
| Traditional Name | ubiquinone 8 |
|---|
| SMILES | COC1=C(OC)C(=O)C(C\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CCC=C(C)C)=C(C)C1=O |
|---|
| InChI Identifier | InChI=1S/C49H74O4/c1-36(2)20-13-21-37(3)22-14-23-38(4)24-15-25-39(5)26-16-27-40(6)28-17-29-41(7)30-18-31-42(8)32-19-33-43(9)34-35-45-44(10)46(50)48(52-11)49(53-12)47(45)51/h20,22,24,26,28,30,32,34H,13-19,21,23,25,27,29,31,33,35H2,1-12H3/b37-22+,38-24+,39-26+,40-28+,41-30+,42-32+,43-34+ |
|---|
| InChI Key | ICFIZJQGJAJRSU-SGHXUWJISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as ubiquinones. These are coenzyme Q derivatives containing a 5, 6-dimethoxy-3-methyl(1,4-benzoquinone) moiety to which an isoprenyl group is attached at ring position 2(or 6). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Quinone and hydroquinone lipids |
|---|
| Direct Parent | Ubiquinones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Polyterpenoid
- Polyprenylbenzoquinone
- Ubiquinone skeleton
- Quinone
- P-benzoquinone
- Vinylogous ester
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0212133900-c86f25544d97c9e8ab2b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0035-0549561100-faeb32bb812d2639f397 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kai-1122291000-6a0ee9e6ff71c52692d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000001900-c46ed8c81a84a99af90f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056r-0100009700-0b318638bf4524a32cf2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4r-8100019300-fbc1eba5fd3761fdc3ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00u2-5614449500-c3a3632765e6fbdda8b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052b-3904312000-e8a2aa378a78c67cf732 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000x-9614300000-6852b0dc4e884ba0a235 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000000900-cb1c91c6236a9d224d54 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004j-0910008400-57fc5f04939c7df4523e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00lr-0392012100-35c8d6743ca8b9a029cf | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0304524 |
|---|
| FooDB ID | FDB031235 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | UBIQUINONE-8 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4446659 |
|---|
| ChEBI ID | 61683 |
|---|
| PubChem Compound ID | 5283546 |
|---|
| Kegg Compound ID | C17569 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | ECMDB21296 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|