| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 02:02:14 UTC |
|---|
| Update Date | 2016-11-09 01:22:40 UTC |
|---|
| Accession Number | CHEM042414 |
|---|
| Identification |
|---|
| Common Name | taxa-4,11-diene |
|---|
| Class | Small Molecule |
|---|
| Description | Taxa-4,11-diene belongs to taxanes and derivatives class of compounds. Those are diterpenoids with a structure based either on the taxane skeleton, or a derivative thereof. In term of phytochemistry, several derivatives of the taxane skeleton exist: 2(3->20)-abeotaxane, 3,11-cyclotaxane, 11(15->1),11(10->9)-abeotaxane, 3,8-seco-taxane, and 11(15->1)-abeotaxane, among others. More complex skeletons have been found recently, which include the taxane-derived [3.3.3] propellane ring system. Taxa-4,11-diene can be found in a number of food items such as peach, tronchuda cabbage, european cranberry, and arrowroot, which makes taxa-4,11-diene a potential biomarker for the consumption of these food products. Enzymatically, taxadiene is produced from geranylgeranyl pyrophosphate by taxadiene synthase. A biochemical gram-scale production of taxadiene has been reported in 2010 using genetically engineered Escherichia coli . |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
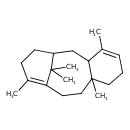
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C20H32 |
|---|
| Average Molecular Mass | 272.476 g/mol |
|---|
| Monoisotopic Mass | 272.250 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 4,8,12,15,15-pentamethyltricyclo[9.3.1.0³,⁸]pentadeca-4,11-diene |
|---|
| Traditional Name | 4,8,12,15,15-pentamethyltricyclo[9.3.1.0³,⁸]pentadeca-4,11-diene |
|---|
| SMILES | CC1=C2CCC3(C)CCC=C(C)C3CC(CC1)C2(C)C |
|---|
| InChI Identifier | InChI=1S/C20H32/c1-14-7-6-11-20(5)12-10-17-15(2)8-9-16(13-18(14)20)19(17,3)4/h7,16,18H,6,8-13H2,1-5H3 |
|---|
| InChI Key | FRJSECSOXKQMOD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as taxanes and derivatives. These are diterpenoids with a structure based either on the taxane skeleton, or a derivative thereof. In term of phytochemistry, several derivatives of the taxane skeleton exist: 2(3->20)-abeotaxane, 3,11-cyclotaxane, 11(15->1),11(10->9)-abeotaxane, 3,8-seco-taxane, and 11(15->1)-abeotaxane, among others. More complex skeletons have been found recently, which include the taxane-derived [3.3.3] propellane ring system. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Diterpenoids |
|---|
| Direct Parent | Taxanes and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Taxane diterpenoid
- Branched unsaturated hydrocarbon
- Polycyclic hydrocarbon
- Cyclic olefin
- Unsaturated aliphatic hydrocarbon
- Unsaturated hydrocarbon
- Olefin
- Hydrocarbon
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0090000000-abf4e7f7a552a168ea9d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-1290000000-6b0bc3213e03a67108df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052s-2590000000-8b140a2d3ff3913c5f44 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-0ceb6eb7b803adfd0761 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0090000000-1f1f9a9cb86d03ef5f5f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4s-0490000000-25aa99204f30a8517bcc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0190000000-1fe73e8deab44fbf66ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0590000000-7b196c5b23ebe2c65440 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fr-0910000000-e79e5b17076483f65a33 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-4c81b77566a3712a2980 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0090000000-4c81b77566a3712a2980 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-0090000000-237649b4d9d7aa69efca | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0304495 |
|---|
| FooDB ID | FDB031191 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Taxadiene |
|---|
| Chemspider ID | 11255339 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 22239694 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|