| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 02:02:09 UTC |
|---|
| Update Date | 2016-11-09 01:22:40 UTC |
|---|
| Accession Number | CHEM042411 |
|---|
| Identification |
|---|
| Common Name | strictosidine |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
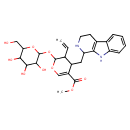
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Methyl 3-ethenyl-4-({1h,2H,3H,4H,9H-pyrido[3,4-b]indol-1-yl}methyl)-2-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,4-dihydro-2H-pyran-5-carboxylic acid | Generator |
|
|---|
| Chemical Formula | C27H34N2O9 |
|---|
| Average Molecular Mass | 530.574 g/mol |
|---|
| Monoisotopic Mass | 530.226 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | methyl 3-ethenyl-4-({1H,2H,3H,4H,9H-pyrido[3,4-b]indol-1-yl}methyl)-2-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,4-dihydro-2H-pyran-5-carboxylate |
|---|
| Traditional Name | methyl 5-ethenyl-4-{1H,2H,3H,4H,9H-pyrido[3,4-b]indol-1-ylmethyl}-6-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-5,6-dihydro-4H-pyran-3-carboxylate |
|---|
| SMILES | COC(=O)C1=COC(OC2OC(CO)C(O)C(O)C2O)C(C=C)C1CC1NCCC2=C1NC1=CC=CC=C21 |
|---|
| InChI Identifier | InChI=1S/C27H34N2O9/c1-3-13-16(10-19-21-15(8-9-28-19)14-6-4-5-7-18(14)29-21)17(25(34)35-2)12-36-26(13)38-27-24(33)23(32)22(31)20(11-30)37-27/h3-7,12-13,16,19-20,22-24,26-33H,1,8-11H2,2H3 |
|---|
| InChI Key | XBAMJZTXGWPTRM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as terpene glycosides. These are prenol lipids containing a carbohydrate moiety glycosidically bound to a terpene backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Terpene glycosides |
|---|
| Direct Parent | Terpene glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Terpene glycoside
- Harman
- Beta-carboline
- Pyridoindole
- Glycosyl compound
- Secoiridoid-skeleton
- O-glycosyl compound
- Aromatic monoterpenoid
- 3-alkylindole
- Monoterpenoid
- Indole
- Indole or derivatives
- Alkaloid or derivatives
- Sugar acid
- Aralkylamine
- Oxane
- Monosaccharide
- Benzenoid
- Heteroaromatic compound
- Vinylogous ester
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Pyrrole
- Methyl ester
- Secondary alcohol
- Amino acid or derivatives
- Carboxylic acid ester
- Polyol
- Secondary amine
- Carboxylic acid derivative
- Organoheterocyclic compound
- Azacycle
- Monocarboxylic acid or derivatives
- Oxacycle
- Acetal
- Secondary aliphatic amine
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Alcohol
- Carbonyl group
- Organic oxygen compound
- Primary alcohol
- Organonitrogen compound
- Organooxygen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-02ai-0569150000-e78847e65f3574bfcb8e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0jbc-0659010000-ead06cff32e3cca1f76f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fen-8896010000-75b80baf5c3cb8ed33f1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00or-1209170000-6e2b5278afd1a514194d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02ti-2309110000-a746efd622f0dcb9d832 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00pl-7489000000-7827fb11562e4e7eebd3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uyi-0109030000-452b4a955968062f65df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0v4i-0129000000-193a6d31aa0a79866d9c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-003i-9705100000-a5ca1393ff5857c611f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-102i-0109010000-c4da51e93b879fb83b99 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052f-3569420000-afb1bda518c0be62955c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-2329000000-965094577f49e5d3fd07 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0304492 |
|---|
| FooDB ID | FDB031186 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 2512293 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 3262516 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|