| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 02:01:45 UTC |
|---|
| Update Date | 2016-11-09 01:22:40 UTC |
|---|
| Accession Number | CHEM042398 |
|---|
| Identification |
|---|
| Common Name | salutaridinol |
|---|
| Class | Small Molecule |
|---|
| Description | Salutaridinol belongs to phenanthrenes and derivatives class of compounds. Those are polycyclic compounds containing a phenanthrene moiety, which is a tricyclic aromatic compound with three non-linearly fused benzene. Salutaridinol is practically insoluble (in water) and a very weakly acidic compound (based on its pKa). Salutaridinol can be found in a number of food items such as pummelo, cardamom, yellow wax bean, and chinese bayberry, which makes salutaridinol a potential biomarker for the consumption of these food products. Salutaridinol is a modified benzyltetrahydroisoquinoline alkaloid with the formula C19H23NO4. It is produced in the secondary metabolism of the opium poppy Papaver somniferum (Papaveraceae) as an intermediate in the biosynthetic pathway that generates morphine. As an isoquinoline alkaloid, it is fundamentally derived from tyrosine as part of the shikimate pathway of secondary metabolism. Salutaridinol is a product of the enzyme salutaridine: NADPH 7-oxidoreductase and the substrate for the enzyme salutaridinol 7-O-acetyltransferase, which are two of the four enzymes in the morphine biosynthesis pathway that generates morphine from (R)-reticuline. Salutaridinol's unique position adjacent to two of the four enzymes in the morphine biosynthesis pathway gives it an important role in enzymatic, genetic, and synthetic biology studies of morphine biosynthesis. Salutaridinol levels are indicative of the flux through the morphine biosynthesis pathway and the efficacy of both salutaridine: NADPH 7-oxidoreductase and salutaridinol 7-O-acetyltransferase . |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
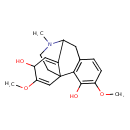
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C19H23NO4 |
|---|
| Average Molecular Mass | 329.396 g/mol |
|---|
| Monoisotopic Mass | 329.163 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 4,13-dimethoxy-17-methyl-17-azatetracyclo[7.5.3.0¹,¹⁰.0²,⁷]heptadeca-2(7),3,5,10,13-pentaene-3,12-diol |
|---|
| Traditional Name | 4,13-dimethoxy-17-methyl-17-azatetracyclo[7.5.3.0¹,¹⁰.0²,⁷]heptadeca-2(7),3,5,10,13-pentaene-3,12-diol |
|---|
| SMILES | COC1=CC23CCN(C)C(CC4=C2C(O)=C(OC)C=C4)C3=CC1O |
|---|
| InChI Identifier | InChI=1S/C19H23NO4/c1-20-7-6-19-10-16(24-3)14(21)9-12(19)13(20)8-11-4-5-15(23-2)18(22)17(11)19/h4-5,9-10,13-14,21-22H,6-8H2,1-3H3 |
|---|
| InChI Key | LLSADFZHWMEBHH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenanthrenes and derivatives. These are polycyclic compounds containing a phenanthrene moiety, which is a tricyclic aromatic compound with three non-linearly fused benzene. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenanthrenes and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Phenanthrenes and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenanthrene
- Benzazocine
- Tetralin
- Anisole
- Alkyl aryl ether
- 1-hydroxy-4-unsubstituted benzenoid
- Aralkylamine
- Piperidine
- Secondary alcohol
- Tertiary amine
- Tertiary aliphatic amine
- Ether
- Azacycle
- Organoheterocyclic compound
- Organic nitrogen compound
- Alcohol
- Hydrocarbon derivative
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0019000000-5d038a616a9cc89ca55c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0059000000-4180d19fbec228fd0300 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002f-0290000000-05823663907c42ba39d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-267f15e47e7d94e5f63a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0039000000-6a148fd32f33c700176c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01x0-1091000000-eb698df300b279760e56 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0009000000-35d228ab3d85d04bd5e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0039000000-5a8192c5ce7433325716 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002g-2093000000-bb438e12d35a0c730546 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-48c9693a0346ebfa2d97 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0039000000-ee2c248d8a92406ef098 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-06tb-0091000000-aa3036a0d79ddffb64e8 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | FDB031165 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Salutaridinol |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 630157 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|