| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 02:00:16 UTC |
|---|
| Update Date | 2016-11-09 01:22:39 UTC |
|---|
| Accession Number | CHEM042375 |
|---|
| Identification |
|---|
| Common Name | pinocembrin chalcone |
|---|
| Class | Small Molecule |
|---|
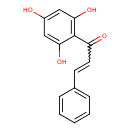
| Description | 3-phenyl-1-(2,4,6-trihydroxyphenyl)prop-2-en-1-one is a member of the class of compounds known as 2'-hydroxychalcones. 2'-hydroxychalcones are organic compounds containing chalcone skeleton that carries a hydroxyl group at the 2'-position. 3-phenyl-1-(2,4,6-trihydroxyphenyl)prop-2-en-1-one is practically insoluble (in water) and a very weakly acidic compound (based on its pKa). 3-phenyl-1-(2,4,6-trihydroxyphenyl)prop-2-en-1-one can be found in a number of food items such as parsley, sesame, poppy, and borage, which makes 3-phenyl-1-(2,4,6-trihydroxyphenyl)prop-2-en-1-one a potential biomarker for the consumption of these food products. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C15H12O4 |
|---|
| Average Molecular Mass | 256.257 g/mol |
|---|
| Monoisotopic Mass | 256.074 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 3-phenyl-1-(2,4,6-trihydroxyphenyl)prop-2-en-1-one |
|---|
| Traditional Name | 3-phenyl-1-(2,4,6-trihydroxyphenyl)prop-2-en-1-one |
|---|
| SMILES | OC1=CC(O)=C(C(=O)C=CC2=CC=CC=C2)C(O)=C1 |
|---|
| InChI Identifier | InChI=1S/C15H12O4/c16-11-8-13(18)15(14(19)9-11)12(17)7-6-10-4-2-1-3-5-10/h1-9,16,18-19H |
|---|
| InChI Key | LOYXTWZXLWHMBX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 2'-hydroxychalcones. These are organic compounds containing chalcone skeleton that carries a hydroxyl group at the 2'-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Linear 1,3-diarylpropanoids |
|---|
| Sub Class | Chalcones and dihydrochalcones |
|---|
| Direct Parent | 2'-Hydroxychalcones |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2'-hydroxychalcone
- Cinnamylphenol
- Acylphloroglucinol derivative
- Benzenetriol
- Phloroglucinol derivative
- Benzoyl
- Aryl ketone
- Styrene
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Acryloyl-group
- Vinylogous acid
- Enone
- Alpha,beta-unsaturated ketone
- Ketone
- Polyol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0pir-3714900000-c7bf531806afaae5d14c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-1950000000-def1c3287bf6876df6c7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0190000000-bafe0b48b0dc2750850a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pb9-0960000000-eb1a10c97860167bc003 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-1900000000-19176f0dc439e40884ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0390000000-a741f6924fe505116e2a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056r-0930000000-739e23fd6a7daffbb2b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fb9-2900000000-1915e79470ca9c3f4da8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0190000000-66d2b703382217bc7172 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0kdl-3940000000-9da4911e603394bfe0a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-8566d45476ba1e73b7df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0590000000-fe7c82a91116f5fb10ff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-1900000000-5edbe48bc6dbc3aef4a8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-4900000000-d2936f396035ad2980b1 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0128077 |
|---|
| FooDB ID | FDB031121 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 460718 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|