| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 02:00:06 UTC |
|---|
| Update Date | 2016-11-09 01:22:39 UTC |
|---|
| Accession Number | CHEM042370 |
|---|
| Identification |
|---|
| Common Name | phosphoadenosine-5'-phosphosulfate |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
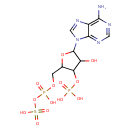
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3'-Phosphonato-5'-adenylyl sulfuric acid(4-) | Generator | | 3'-Phosphonato-5'-adenylyl sulphate(4-) | Generator | | 3'-Phosphonato-5'-adenylyl sulphuric acid(4-) | Generator | | 3'-Phosphoadenylyl sulfate | HMDB | | 3'-Phosphonato-5'-adenylyl sulfate | HMDB | | 3'-Phosphonato-5'-adenylyl sulfate tetraanion | HMDB | | 3'-Phosphonatoadenosine 5'-phosphosulfate | HMDB | | 3'-Phosphonatoadenosine 5'-phosphosulfate tetraanion | HMDB | | 3'-Phosphonatoadenosine 5'-phosphosulfate(4-) | HMDB | | PAPS | HMDB | | PAPS tetraanion | HMDB | | PAPS(4-) | HMDB | | 3'-Phosphoadenylyl sulfuric acid | HMDB | | 3'-Phosphoadenylyl sulphate | HMDB | | 3'-Phosphoadenylyl sulphuric acid | HMDB | | 3'-Phosphonato-5'-adenylyl sulfuric acid | HMDB | | 3'-Phosphonato-5'-adenylyl sulphate | HMDB | | 3'-Phosphonato-5'-adenylyl sulphuric acid | HMDB | | 3'-Phosphonato-5'-adenylyl sulfuric acid tetraanion | HMDB | | 3'-Phosphonato-5'-adenylyl sulphate tetraanion | HMDB | | 3'-Phosphonato-5'-adenylyl sulphuric acid tetraanion | HMDB | | 3'-Phosphonatoadenosine 5'-phosphosulfuric acid | HMDB | | 3'-Phosphonatoadenosine 5'-phosphosulphate | HMDB | | 3'-Phosphonatoadenosine 5'-phosphosulphuric acid | HMDB | | 3'-Phosphonatoadenosine 5'-phosphosulfuric acid tetraanion | HMDB | | 3'-Phosphonatoadenosine 5'-phosphosulphate tetraanion | HMDB | | 3'-Phosphonatoadenosine 5'-phosphosulphuric acid tetraanion | HMDB | | 3'-Phosphonatoadenosine 5'-phosphosulfuric acid(4-) | HMDB | | 3'-Phosphonatoadenosine 5'-phosphosulphate(4-) | HMDB | | 3'-Phosphonatoadenosine 5'-phosphosulphuric acid(4-) | HMDB | | Phosphoadenosine phosphosulfate | HMDB | | Phosphosulfate, phosphoadenosine | HMDB | | Adenosine-3'-phosphate-5'-phosphosulfate | HMDB | | Adenosine 3' phosphate 5' phosphosulfate | HMDB |
|
|---|
| Chemical Formula | C10H11N5O13P2S |
|---|
| Average Molecular Mass | 503.230 g/mol |
|---|
| Monoisotopic Mass | 502.957 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | [({[5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]sulfonic acid |
|---|
| Traditional Name | {[5-(6-aminopurin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy(hydroxy)phosphoryl}oxysulfonic acid |
|---|
| SMILES | [H][C@]1(COP([O-])(=O)OS([O-])(=O)=O)O[C@@]([H])(N2C=NC3=C(N)N=CN=C23)[C@]([H])(O)[C@]1([H])OP([O-])([O-])=O |
|---|
| InChI Identifier | InChI=1S/C10H15N5O13P2S/c11-8-5-9(13-2-12-8)15(3-14-5)10-6(16)7(27-29(17,18)19)4(26-10)1-25-30(20,21)28-31(22,23)24/h2-4,6-7,10,16H,1H2,(H,20,21)(H2,11,12,13)(H2,17,18,19)(H,22,23,24)/p-4/t4-,6-,7-,10-/m1/s1 |
|---|
| InChI Key | GACDQMDRPRGCTN-KQYNXXCUSA-J |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sugar acids and derivatives. Sugar acids and derivatives are compounds containing a saccharide unit which bears a carboxylic acid group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Sugar acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sugar acid
- Short-chain hydroxy acid
- Beta-hydroxy acid
- Fatty acid
- Monosaccharide
- Hydroxy acid
- Dicarboxylic acid or derivatives
- Alpha-hydroxy acid
- Secondary alcohol
- 1,2-diol
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-2901310000-a33e993c071bb792b6f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0900100000-91538fd4e2b64f203e05 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-3900000000-f96ffb936f26966b7a51 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a7i-4900150000-72bfa7da97da01f8a1a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-003r-6900000000-c33a5254e78e91e7e0b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9300000000-cac8848466e032b4a3ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000090000-ba006402f6f0af41d779 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ri-0402920000-7d549a97dd2276b8b754 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01qi-0439100000-94b8e2004e4fb012c91d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0900000000-63709bad7395f9f33acc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0900000000-94a8fdc8360cf2577ca6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-003s-8900000000-0b1152e8f0b7373e9349 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0062646 |
|---|
| FooDB ID | FDB034848 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 990 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|