| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:57:58 UTC |
|---|
| Update Date | 2016-11-09 01:22:39 UTC |
|---|
| Accession Number | CHEM042302 |
|---|
| Identification |
|---|
| Common Name | magnesium-protoporphyrin IX 13-monomethyl ester |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
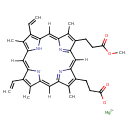
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C35H34MgN4O4 |
|---|
| Average Molecular Mass | 598.986 g/mol |
|---|
| Monoisotopic Mass | 598.243 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | magnesium(2+) ion 14-(2-carboxylatoethyl)-5,20-diethenyl-10-(3-methoxy-3-oxopropyl)-4,9,15,19-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1³,⁶.1⁸,¹¹.1¹³,¹⁶]tetracosa-1,3,5,7,9,11(23),12,14,16(22),17,19-undecaen-21-ide |
|---|
| Traditional Name | magnesium(2+) ion 14-(2-carboxylatoethyl)-5,20-diethenyl-10-(3-methoxy-3-oxopropyl)-4,9,15,19-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1³,⁶.1⁸,¹¹.1¹³,¹⁶]tetracosa-1,3,5,7,9,11(23),12,14,16(22),17,19-undecaen-21-ide |
|---|
| SMILES | [Mg++].COC(=O)CCC1=C(C)\C2=C\C3=C(C=C)C(C)=C(N3)\C=C3/[N-]\C(=C/C4=N/C(=C\C1=N2)/C(CCC([O-])=O)=C4C)C(C)=C3C=C |
|---|
| InChI Identifier | InChI=1S/C35H36N4O4.Mg/c1-8-22-18(3)26-14-27-20(5)24(10-12-34(40)41)32(38-27)17-33-25(11-13-35(42)43-7)21(6)29(39-33)16-31-23(9-2)19(4)28(37-31)15-30(22)36-26;/h8-9,14-17H,1-2,10-13H2,3-7H3,(H3,36,37,38,39,40,41);/q;+2/p-2/b26-14-,27-14-,28-15-,29-16-,30-15-,31-16-,32-17-,33-17-; |
|---|
| InChI Key | URVKSRIHRHKVRW-NCCDZXNNSA-L |
|---|
| Chemical Taxonomy |
|---|
| Classification | Not classified |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0000090000-328f993229928488600e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0000090000-328f993229928488600e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-0000090000-328f993229928488600e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000090000-3fd35792c25066bf0501 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0000090000-3fd35792c25066bf0501 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-0000090000-3fd35792c25066bf0501 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|