| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:57:05 UTC |
|---|
| Update Date | 2016-11-09 01:22:38 UTC |
|---|
| Accession Number | CHEM042277 |
|---|
| Identification |
|---|
| Common Name | isochorismate |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
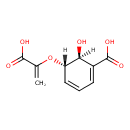
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Isochorismate | Generator | | 5-((1-Carboxyethenyl)oxy)-6-hydroxy-1,3-cyclohexadiene-1-carboxylic acid | MeSH | | Iso-chorismic acid | MeSH | | Isochorismic acid | KEGG |
|
|---|
| Chemical Formula | C10H10O6 |
|---|
| Average Molecular Mass | 226.183 g/mol |
|---|
| Monoisotopic Mass | 226.048 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (5S,6S)-5-[(1-carboxyeth-1-en-1-yl)oxy]-6-hydroxycyclohexa-1,3-diene-1-carboxylic acid |
|---|
| Traditional Name | isochorismic acid |
|---|
| SMILES | [H][C@]1(O)C(=CC=C[C@]1([H])OC(=C)C(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C10H10O6/c1-5(9(12)13)16-7-4-2-3-6(8(7)11)10(14)15/h2-4,7-8,11H,1H2,(H,12,13)(H,14,15)/t7-,8-/m0/s1 |
|---|
| InChI Key | NTGWPRCCOQCMGE-YUMQZZPRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as beta hydroxy acids and derivatives. Beta hydroxy acids and derivatives are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Beta hydroxy acids and derivatives |
|---|
| Direct Parent | Beta hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Beta-hydroxy acid
- Dicarboxylic acid or derivatives
- Secondary alcohol
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | - 5-[(1-carboxyethenyl)oxy]-6-hydroxycyclohexa-1,3-diene-1-carboxylic acid (CHEBI:17582 )
|
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0390000000-442c352acb8b551f3122 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4r-2930000000-ce3efa1e6faec0da376b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-059i-9800000000-340536fab24636b175d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-2790000000-f3ace46a88beae78abf7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a5i-2920000000-ab6fd49186f9e64db922 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-06rl-6900000000-d2314c10e3a03441539f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dr-0900000000-241fe41a6cb87fa3a745 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0079-0900000000-3ca3dca83527af19b555 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0536-9300000000-f0bcd9ed15657be962ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-053i-1900000000-6d5bafcfd1495f0951c1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08g3-2900000000-24982517f03249357ae9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01pc-4900000000-9d508a5a0ed2c89cc844 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0304395 |
|---|
| FooDB ID | FDB030944 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00000734 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 164276 |
|---|
| ChEBI ID | 17582 |
|---|
| PubChem Compound ID | 189062 |
|---|
| Kegg Compound ID | C00885 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | ECMDB20162 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|