| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:54:51 UTC |
|---|
| Update Date | 2016-11-09 01:22:37 UTC |
|---|
| Accession Number | CHEM042216 |
|---|
| Identification |
|---|
| Common Name | dTDP-4-dehydro-6-deoxy-β-L-mannose |
|---|
| Class | Small Molecule |
|---|
| Description | A dTDP-sugar having 4-dehydro-beta-L-rhamnose as the sugar component. It is an intermediate in dTDP-rhamnose biosynthesis. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
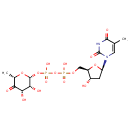
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| dTDP-4-KETO-L-rhamnose | ChEBI | | dTDP-4-oxo-6-Deoxy-L-mannose | ChEBI | | dTDP-4-oxo-L-Rhamnose | ChEBI | | dTDP-6-Deoxy-beta-L-lyxo-hex-4-ulose | ChEBI | | dTDP-4-Dehydro-6-deoxy-beta-L-mannose | Kegg | | dTDP-6-Deoxy-b-L-lyxo-hex-4-ulose | Generator | | dTDP-6-Deoxy-β-L-lyxo-hex-4-ulose | Generator | | dTDP-4-Dehydro-6-deoxy-b-L-mannose | Generator | | dTDP-4-Dehydro-6-deoxy-β-L-mannose | Generator | | dTDP-4-Dehydro-b-L-rhamnose | Generator | | dTDP-4-Dehydro-β-L-rhamnose | Generator |
|
|---|
| Chemical Formula | C16H24N2O15P2 |
|---|
| Average Molecular Mass | 546.314 g/mol |
|---|
| Monoisotopic Mass | 546.065 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | {[(2R,3R,4S,6S)-3,4-dihydroxy-6-methyl-5-oxooxan-2-yl]oxy}({[hydroxy({[(2R,3S,5R)-3-hydroxy-5-(5-methyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl)oxolan-2-yl]methoxy})phosphoryl]oxy})phosphinic acid |
|---|
| Traditional Name | dtdp-4-keto-L-rhamnose |
|---|
| SMILES | C[C@@H]1O[C@H](OP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H](C[C@@H]2O)N2C=C(C)C(=O)NC2=O)[C@H](O)[C@H](O)C1=O |
|---|
| InChI Identifier | InChI=1S/C16H24N2O15P2/c1-6-4-18(16(24)17-14(6)23)10-3-8(19)9(31-10)5-29-34(25,26)33-35(27,28)32-15-13(22)12(21)11(20)7(2)30-15/h4,7-10,12-13,15,19,21-22H,3,5H2,1-2H3,(H,25,26)(H,27,28)(H,17,23,24)/t7-,8-,9+,10+,12+,13+,15+/m0/s1 |
|---|
| InChI Key | PSXWNITXWWECNY-LPVGZGSHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as kaurane diterpenoids. These are diterpene alkaloids with a structure that is based on the kaurane skeleton. Kaurane is a tetracyclic compound that arises by cyclisation of a pimarane precursor followed by rearrangement. It possesses a [3,2,1]-bicyclic ring system with C15-C16 bridge connected to C13, forming the five-membered ring D. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Diterpenoids |
|---|
| Direct Parent | Kaurane diterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Kaurane diterpenoid
- Cyclic alcohol
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_32) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0900000000-d8bcab3cb69bb9b31977 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-3911000000-e257b0c304ce6807e2ed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-4900000000-ad5cfef5709e8b291915 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f6t-3303290000-7fe9024fb90fd086b54e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0w4l-8905010000-ce0f223b98fc4e83f4b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6u-5901000000-b3d378b57fb833ddd965 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0301090000-2b178203d01f87884f2e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-9864440000-97fb5055e9291bb118ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05di-9510000000-03d88eb4122699f5460b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000090000-7f4d40042dbc95c44622 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056r-9404310000-f137c2cfff47fd5ef4e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-2309100000-9ccb4e49f4478067383b | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0304343 |
|---|
| FooDB ID | FDB030837 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 391477 |
|---|
| ChEBI ID | 45868 |
|---|
| PubChem Compound ID | 443211 |
|---|
| Kegg Compound ID | C00688 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|