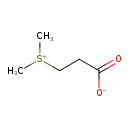
| 3-Dimethylsulfoniopropionate | ChEBI |
| beta-Dimethylsulfoniopropionate | ChEBI |
| Dimethyl-beta-propiothetin | ChEBI |
| Dimethylpropiothetin | ChEBI |
| DMPT | ChEBI |
| DMSP | ChEBI |
| S-Dimethylsulfonium propionic acid | ChEBI |
| 3-Dimethylsulfoniopropionic acid | Generator |
| 3-Dimethylsulphoniopropionate | Generator |
| 3-Dimethylsulphoniopropionic acid | Generator |
| b-Dimethylsulfoniopropionate | Generator |
| b-Dimethylsulfoniopropionic acid | Generator |
| b-Dimethylsulphoniopropionate | Generator |
| b-Dimethylsulphoniopropionic acid | Generator |
| beta-Dimethylsulfoniopropionic acid | Generator |
| beta-Dimethylsulphoniopropionate | Generator |
| beta-Dimethylsulphoniopropionic acid | Generator |
| Β-dimethylsulfoniopropionate | Generator |
| Β-dimethylsulfoniopropionic acid | Generator |
| Β-dimethylsulphoniopropionate | Generator |
| Β-dimethylsulphoniopropionic acid | Generator |
| Dimethyl-b-propiothetin | Generator |
| Dimethyl-β-propiothetin | Generator |
| S-Dimethylsulfonium propionate | Generator |
| S-Dimethylsulphonium propionate | Generator |
| S-Dimethylsulphonium propionic acid | Generator |
| Dimethylsulfoniopropionic acid | Generator |
| Dimethylsulphoniopropionate | Generator |
| Dimethylsulphoniopropionic acid | Generator |
| (2-Carboxyethyl)dimethylsulfonium chloride | MeSH |
| S,S-Dimethyl-beta-propiothetin | MeSH |
| beta-DMSP | MeSH |
| Dimethyl-beta-propiothetin chloride | MeSH |
| Dimethyl-propiothetin | MeSH |
| Dimethylpropiothetin chloride | MeSH |
| Dimethylpropiothetin hydrochloride | MeSH |
| Dimethylsulfoniopropionate chloride | MeSH |
| Sulfonium, (2-carboxyethyl)dimethyl-, chloride (1:1) | MeSH |