| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:53:52 UTC |
|---|
| Update Date | 2016-11-09 01:22:37 UTC |
|---|
| Accession Number | CHEM042178 |
|---|
| Identification |
|---|
| Common Name | cyclo-dopa 5-O-glucoside |
|---|
| Class | Small Molecule |
|---|
| Description | An indolyl carbohydrate that is cyclodopa in which the phenolic hydrogen at position 5 has been replaced by a beta-D-glucosyl residue. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
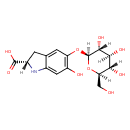
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Cyclo-dopa 5-O-glucoside | ChEBI | | Cyclodopa glucoside | ChEBI | | Leucodopachrome 5-beta-D-glucoside | ChEBI | | Leucodopachrome glucoside | ChEBI | | Leucodopachrome 5-b-D-glucoside | Generator | | Leucodopachrome 5-β-D-glucoside | Generator | | Cyclodopa 5-b-D-glucoside | Generator | | Cyclodopa 5-β-D-glucoside | Generator |
|
|---|
| Chemical Formula | C15H19NO9 |
|---|
| Average Molecular Mass | 357.315 g/mol |
|---|
| Monoisotopic Mass | 357.106 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2S)-6-hydroxy-5-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-2,3-dihydro-1H-indole-2-carboxylic acid |
|---|
| Traditional Name | (2S)-6-hydroxy-5-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-2,3-dihydro-1H-indole-2-carboxylic acid |
|---|
| SMILES | [H][C@]1(CC2=CC(O[C@]3([H])O[C@]([H])(CO)[C@@]([H])(O)[C@]([H])(O)[C@@]3([H])O)=C(O)C=C2N1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C15H19NO9/c17-4-10-11(19)12(20)13(21)15(25-10)24-9-2-5-1-7(14(22)23)16-6(5)3-8(9)18/h2-3,7,10-13,15-21H,1,4H2,(H,22,23)/t7-,10+,11+,12-,13+,15+/m0/s1 |
|---|
| InChI Key | PXMFPPFHRQZIHO-OLCQZNMOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenolic glycosides. These are organic compounds containing a phenolic structure attached to a glycosyl moiety. Some examples of phenolic structures include lignans, and flavonoids. Among the sugar units found in natural glycosides are D-glucose, L-Fructose, and L rhamnose. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Phenolic glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenolic glycoside
- Indolecarboxylic acid derivative
- Indolecarboxylic acid
- Hexose monosaccharide
- O-glycosyl compound
- Alpha-amino acid
- L-alpha-amino acid
- Alpha-amino acid or derivatives
- Indole or derivatives
- Dihydroindole
- Aralkylamine
- Secondary aliphatic/aromatic amine
- 1-hydroxy-2-unsubstituted benzenoid
- Monosaccharide
- Oxane
- Benzenoid
- Amino acid
- Secondary alcohol
- Amino acid or derivatives
- Acetal
- Carboxylic acid derivative
- Carboxylic acid
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Secondary amine
- Monocarboxylic acid or derivatives
- Polyol
- Organic nitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Amine
- Organic oxide
- Carbonyl group
- Primary alcohol
- Alcohol
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-054n-0907000000-ff945066b5dac54a8851 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0092-0900000000-9f1c2cdc65b45467ccb8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006t-0900000000-ca869b2f092a2bb9a36d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4l-0719000000-647a4cd4f5968af93fd7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-1912000000-641aa4739b3647c5c79e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0007-2900000000-81ef127f679a51a2e92c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052b-0904000000-6b9bd6edba3a4a0de741 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0900000000-c5b4821a801403a2095c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k95-3940000000-6e1aff2ef0b4bc6428a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0409000000-805787cb1e9657ecdc29 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f6w-0911000000-c480ac2d6e13f0464653 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002b-0900000000-fc7bd783bfc0525f45d6 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0304310 |
|---|
| FooDB ID | FDB030765 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00053126 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-8656 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30791580 |
|---|
| ChEBI ID | 134458 |
|---|
| PubChem Compound ID | 46173990 |
|---|
| Kegg Compound ID | C17751 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|