| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:52:16 UTC |
|---|
| Update Date | 2016-11-09 01:22:37 UTC |
|---|
| Accession Number | CHEM042137 |
|---|
| Identification |
|---|
| Common Name | butanoyl-CoA |
|---|
| Class | Small Molecule |
|---|
| Description | A short-chain fatty acyl-CoA that results from the formal condensation of the thiol group of coenzyme A with the carboxy group of butyric acid. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
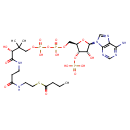
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4:0-CoA | ChEBI | | Butanoyl-CoA | ChEBI | | Butanoyl-coenzyme A | ChEBI | | Butyryl-coenzyme A | ChEBI | | C4:0-CoA | ChEBI | | coenzyme A, S-Butanoate | ChEBI | | S-Butanoyl-CoA | ChEBI | | S-Butanoyl-coenzyme A | ChEBI | | S-Butyryl-CoA | ChEBI | | S-Butyryl-coenzym-a | ChEBI | | S-Butyryl-coenzyme A | ChEBI | | coenzyme A, S-Butanoic acid | Generator | | Butyryl-CoA | MeSH, KEGG |
|
|---|
| Chemical Formula | C25H42N7O17P3S |
|---|
| Average Molecular Mass | 837.624 g/mol |
|---|
| Monoisotopic Mass | 837.157 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2R)-4-({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-N-(2-{[2-(butanoylsulfanyl)ethyl]-C-hydroxycarbonimidoyl}ethyl)-2-hydroxy-3,3-dimethylbutanimidic acid |
|---|
| Traditional Name | (2R)-4-[({[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxy]-N-(2-{[2-(butanoylsulfanyl)ethyl]-C-hydroxycarbonimidoyl}ethyl)-2-hydroxy-3,3-dimethylbutanimidic acid |
|---|
| SMILES | [H][C@](O)(C(O)=NCCC(O)=NCCSC(=O)CCC)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@@]1([H])O[C@@]([H])(N2C=NC3=C(N)N=CN=C23)[C@]([H])(O)[C@]1([H])OP(O)(O)=O |
|---|
| InChI Identifier | InChI=1S/C25H42N7O17P3S/c1-4-5-16(34)53-9-8-27-15(33)6-7-28-23(37)20(36)25(2,3)11-46-52(43,44)49-51(41,42)45-10-14-19(48-50(38,39)40)18(35)24(47-14)32-13-31-17-21(26)29-12-30-22(17)32/h12-14,18-20,24,35-36H,4-11H2,1-3H3,(H,27,33)(H,28,37)(H,41,42)(H,43,44)(H2,26,29,30)(H2,38,39,40)/t14-,18-,19-,20+,24-/m1/s1 |
|---|
| InChI Key | CRFNGMNYKDXRTN-CITAKDKDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dihydroxy bile acids, alcohols and derivatives. Dihydroxy bile acids, alcohols and derivatives are compounds containing or derived from a bile acid or alcohol, and which bears exactly two carboxylic acid groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Dihydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Ergosterol-skeleton
- Ergostane-skeleton
- Ecdysteroid
- Dihydroxy bile acid, alcohol, or derivatives
- 22-hydroxysteroid
- 3-hydroxysteroid
- 6-oxosteroid
- Oxosteroid
- Hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Ketone
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-017i-7930142460-c2298a969e995ad3d00a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-5910100010-7599cb988facd52223da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-057i-6900100000-f5f10442ccac27dc38eb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000000090-073f428b330788172433 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014r-9200102230-8569a6d934e6ef361838 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0170-4003402900-aa858b6cf989ef7545e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1931000110-da1db28a855c878b4aed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000j-1921000000-5675ad3c288d42128697 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ks-2910000000-64ab778c97047046a2d9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0000000190-722be0a11d66c5eb058c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0911001570-3a8be9989cbf457b7031 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-0019000000-35adb55601f58e5ce0ac | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0304279 |
|---|
| FooDB ID | FDB030704 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 109043 |
|---|
| ChEBI ID | 15517 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C00136 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | ECMDB01088 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|