| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:49:36 UTC |
|---|
| Update Date | 2016-11-09 01:22:36 UTC |
|---|
| Accession Number | CHEM042094 |
|---|
| Identification |
|---|
| Common Name | 7,8-dihydromonapterin |
|---|
| Class | Small Molecule |
|---|
| Description | A dihydropterin that is monapterin dihydrogenated at positions 7 and 8. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
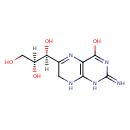
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| DHM | ChEBI | | H2-MPT | ChEBI |
|
|---|
| Chemical Formula | C9H13N5O4 |
|---|
| Average Molecular Mass | 255.231 g/mol |
|---|
| Monoisotopic Mass | 255.097 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (1S,2S)-1-(4-hydroxy-2-imino-1,2,7,8-tetrahydropteridin-6-yl)propane-1,2,3-triol |
|---|
| Traditional Name | (1S,2S)-1-(4-hydroxy-2-imino-7,8-dihydro-1H-pteridin-6-yl)propane-1,2,3-triol |
|---|
| SMILES | [H][C@](O)(CO)[C@@]([H])(O)C1=NC2=C(NC1)NC(=N)N=C2O |
|---|
| InChI Identifier | InChI=1S/C9H13N5O4/c10-9-13-7-5(8(18)14-9)12-3(1-11-7)6(17)4(16)2-15/h4,6,15-17H,1-2H2,(H4,10,11,13,14,18)/t4-,6-/m0/s1 |
|---|
| InChI Key | YQIFAMYNGGOTFB-NJGYIYPDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as biopterins and derivatives. These are coenzymes containing a 2-amino-pteridine-4-one derivative. They are mainly synthesized in several parts of the body, including the pineal gland. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
| Direct Parent | Biopterins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Biopterin
- Aminopyrimidine
- Pyrimidone
- Secondary aliphatic/aromatic amine
- Pyrimidine
- Vinylogous amide
- Heteroaromatic compound
- Ketimine
- Secondary alcohol
- Azacycle
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Secondary amine
- Polyol
- Amine
- Organopnictogen compound
- Primary amine
- Primary alcohol
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Imine
- Organic oxygen compound
- Organic nitrogen compound
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_5_10) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0090000000-2b0b342a426dd07d904d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01p9-1790000000-6794185a47369434a21d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03k9-3900000000-07c24d178dd8bbca5295 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udl-0290000000-989ec6a0993db433af7e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0gvo-3950000000-ff612a77e1e2c8134d4c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9300000000-07fb79f2bfe6d05bf124 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-5310e77f7d773306f577 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-066r-0590000000-b180036f6a8d6a15b2e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00fv-1910000000-c73199c0d2c4515991d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01ox-0920000000-ebf1b5bf4dbba9d0f07f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01r6-0900000000-b8bb6081bbc0df64ad18 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014u-9810000000-20e6aa6d596718131072 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0304238 |
|---|
| FooDB ID | FDB030618 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-11770 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 71175 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|