| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:45:52 UTC |
|---|
| Update Date | 2016-11-09 01:22:34 UTC |
|---|
| Accession Number | CHEM041952 |
|---|
| Identification |
|---|
| Common Name | 3-(all-trans-octaprenyl)benzene-1,2-diol |
|---|
| Class | Small Molecule |
|---|
| Description | A 3-(all-trans-polyprenyl)benzene-1,2-diol in which the substituent at position 3 is an all-trans-octaprenyl moiety. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
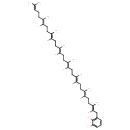
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Octaprenyl-6-hydroxyphenol | ChEBI | | 3-Octaprenylcatechol | ChEBI |
|
|---|
| Chemical Formula | C46H70O2 |
|---|
| Average Molecular Mass | 655.064 g/mol |
|---|
| Monoisotopic Mass | 654.538 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 3-[(2E,6E,10E,14E,18E,22E,26E)-3,7,11,15,19,23,27,31-octamethyldotriaconta-2,6,10,14,18,22,26,30-octaen-1-yl]benzene-1,2-diol |
|---|
| Traditional Name | 2-octaprenyl-6-hydroxyphenol |
|---|
| SMILES | [H]\C(CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC1=C(O)C(O)=CC=C1)=C(\C)CCC=C(C)C |
|---|
| InChI Identifier | InChI=1S/C46H70O2/c1-36(2)18-10-19-37(3)20-11-21-38(4)22-12-23-39(5)24-13-25-40(6)26-14-27-41(7)28-15-29-42(8)30-16-31-43(9)34-35-44-32-17-33-45(47)46(44)48/h17-18,20,22,24,26,28,30,32-34,47-48H,10-16,19,21,23,25,27,29,31,35H2,1-9H3/b37-20+,38-22+,39-24+,40-26+,41-28+,42-30+,43-34+ |
|---|
| InChI Key | YNPGYMZVNLIZLD-BQFKTQOQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as polyprenylbenzene-1,2-diols. Polyprenylbenzene-1,2-diols are compounds containing a polyisoprene chain attached to a catechol group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Polyprenylphenols |
|---|
| Direct Parent | Polyprenylbenzene-1,2-diols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetraterpenoid
- Polyprenylbenzene-1,2-diol
- Catechol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Benzenoid
- Monocyclic benzene moiety
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | - 3-(all-trans-polyprenyl)benzene-1,2-diol (CHEBI:1233 )
|
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0312129000-8437c33863881ba7cea8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00c0-1649470000-47757fd3ad962efe01fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053s-2569570000-1c4e1b069dfcdcd925ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000009000-a53175b2cb988cc5146b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0100019000-c057a57e9add40db6431 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052r-1512159000-823d19cfee76969e3a65 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0awj-0101194000-b5bbec2d16e420258dac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-009l-2013692000-6571234a6177920be7b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05di-0837980000-9f0d8c8c654814d9f342 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000009000-1be28d88036bf99e5c80 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0210129000-fcd884fe58e375a3d4c7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a5i-0912312000-a591ae46754aa6bd1a86 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0304113 |
|---|
| FooDB ID | FDB030400 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | 2-OCTAPRENYL-6-HYDROXYPHENOL |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4444382 |
|---|
| ChEBI ID | 1233 |
|---|
| PubChem Compound ID | 5280833 |
|---|
| Kegg Compound ID | C05811 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | ECMDB04117 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|