| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:43:33 UTC |
|---|
| Update Date | 2016-11-09 01:22:33 UTC |
|---|
| Accession Number | CHEM041858 |
|---|
| Identification |
|---|
| Common Name | 16-epivellosimine |
|---|
| Class | Small Molecule |
|---|
| Description | An indole alkaloid that is vellosimine in which the carbon bearing the aldehyde function has been epimerised. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
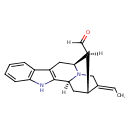
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 16-Epi-vellosimine | MeSH |
|
|---|
| Chemical Formula | C19H20N2O |
|---|
| Average Molecular Mass | 292.375 g/mol |
|---|
| Monoisotopic Mass | 292.158 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (1S,12S,13S,14R,15E)-15-ethylidene-3,17-diazapentacyclo[12.3.1.0^{2,10}.0^{4,9}.0^{12,17}]octadeca-2(10),4(9),5,7-tetraene-13-carbaldehyde |
|---|
| Traditional Name | (1S,12S,13S,14R,15E)-15-ethylidene-3,17-diazapentacyclo[12.3.1.0^{2,10}.0^{4,9}.0^{12,17}]octadeca-2(10),4(9),5,7-tetraene-13-carbaldehyde |
|---|
| SMILES | [H]\C(C)=C1/CN2[C@@]3([H])C[C@]1([H])[C@]([H])(C=O)[C@]2([H])CC1=C3NC2=CC=CC=C12 |
|---|
| InChI Identifier | InChI=1S/C19H20N2O/c1-2-11-9-21-17-8-14-12-5-3-4-6-16(12)20-19(14)18(21)7-13(11)15(17)10-22/h2-6,10,13,15,17-18,20H,7-9H2,1H3/b11-2-/t13-,15-,17-,18-/m0/s1 |
|---|
| InChI Key | MHASSCPGKAMILD-MIOJWWSHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as macroline alkaloids. These are alkaloids with a structure that is based on the tetracyclic macroline skeleton. The macroline skeleton arises by scission of the C-21 to N-4 bond of the akuammilan skeleton, and mostly occurs in bisindole alkaloids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Macroline alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Macroline alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Macroline skeleton
- Sarpagine-skeleton
- Vobasan skeleton
- Beta-carboline
- Pyridoindole
- 3-alkylindole
- Indole
- Indole or derivatives
- Quinuclidine
- Aralkylamine
- Benzenoid
- Piperidine
- Heteroaromatic compound
- Pyrrole
- Tertiary aliphatic amine
- Tertiary amine
- Organoheterocyclic compound
- Azacycle
- Organic nitrogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Amine
- Aldehyde
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-502966b71b3fa4b51088 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0090000000-4071f18f2e91b42c21ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0059-2980000000-df284da1af082841e13d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-eb9636b759144711f704 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ox-0090000000-566d1bb0322a9b8a7e90 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-0090000000-6466f92d5f8fff4b027f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-30850ca9637e3e228dfd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0090000000-d3385634a3967ef0b42c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-072a-0190000000-27d6f13478b5896b1cf5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-74a3146908205cd6ebec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0090000000-972804a8372b111e10d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dr-0090000000-59d95f3c81fe9761c0cc | Spectrum | | MS | Mass Spectrum (Electron Ionization) | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0304026 |
|---|
| FooDB ID | FDB030270 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00051789 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | 16-EPIVELLOSIMINE |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 9510274 |
|---|
| ChEBI ID | 16425 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C11633 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|