| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:40:21 UTC |
|---|
| Update Date | 2016-11-09 01:22:31 UTC |
|---|
| Accession Number | CHEM041722 |
|---|
| Identification |
|---|
| Common Name | (22α)-hydroxy-campesterol |
|---|
| Class | Small Molecule |
|---|
| Description | (22alpha)-hydroxy-campesterol is practically insoluble (in water) and an extremely weak acidic compound (based on its pKa). (22alpha)-hydroxy-campesterol can be found in a number of food items such as plains prickly pear, european cranberry, roman camomile, and caraway, which makes (22alpha)-hydroxy-campesterol a potential biomarker for the consumption of these food products. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
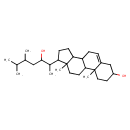
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (22a)-Hydroxy-campesterol | Generator | | (22Α)-hydroxy-campesterol | Generator |
|
|---|
| Chemical Formula | C28H48O2 |
|---|
| Average Molecular Mass | 416.690 g/mol |
|---|
| Monoisotopic Mass | 416.365 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 14-(3-hydroxy-5,6-dimethylheptan-2-yl)-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-5-ol |
|---|
| Traditional Name | 14-(3-hydroxy-5,6-dimethylheptan-2-yl)-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-5-ol |
|---|
| SMILES | CC(C)C(C)CC(O)C(C)C1CCC2C3CC=C4CC(O)CCC4(C)C3CCC12C |
|---|
| InChI Identifier | InChI=1S/C28H48O2/c1-17(2)18(3)15-26(30)19(4)23-9-10-24-22-8-7-20-16-21(29)11-13-27(20,5)25(22)12-14-28(23,24)6/h7,17-19,21-26,29-30H,8-16H2,1-6H3 |
|---|
| InChI Key | LSZJAIFORSLKOY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dihydroxy bile acids, alcohols and derivatives. Dihydroxy bile acids, alcohols and derivatives are compounds containing or derived from a bile acid or alcohol, and which bears exactly two carboxylic acid groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Dihydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Ergostane-skeleton
- Ergosterol-skeleton
- Dihydroxy bile acid, alcohol, or derivatives
- 22-hydroxysteroid
- Hydroxysteroid
- 3-hydroxysteroid
- 3-hydroxy-delta-5-steroid
- Delta-5-steroid
- Cyclic alcohol
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kb-0009200000-86fc5c523c9376a5beb2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01qj-2019100000-3029c3b4b55bc1c54839 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k9i-9067100000-a55cc69c9a54a4a31e36 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0003900000-a540c5ba54842bc05358 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014j-2009800000-99aeafbdc956037b7bc3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f7a-6149000000-df07b97e3077625542d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-4317900000-4ec1d750bf3813bcc82b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002e-9225100000-45f7abdd31fb8e4b9f36 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-6920000000-b42dab28a88a0274df60 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000900000-a74b65a822c3cc7625b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0002900000-3a514d2a716642544785 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0hh9-1039600000-d3065783d6b7de18cfec | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0303957 |
|---|
| FooDB ID | FDB030097 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 24784761 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 73192390 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|