| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:36:28 UTC |
|---|
| Update Date | 2016-11-09 01:22:31 UTC |
|---|
| Accession Number | CHEM041651 |
|---|
| Identification |
|---|
| Common Name | trans-Resveratrol 3,4'-disulfate |
|---|
| Class | Small Molecule |
|---|
| Description | trans-Resveratrol 3,4'-disulfate is a polyphenol metabolite detected in biological fluids (PMID: 20428313). |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
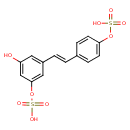
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| trans-Resveratrol 3,4'-disulfuric acid | Generator | | trans-Resveratrol 3,4'-disulphate | Generator | | trans-Resveratrol 3,4'-disulphuric acid | Generator | | {4-[(e)-2-[3-hydroxy-5-(sulfooxy)phenyl]ethenyl]phenyl}oxidanesulfonate | HMDB | | {4-[(e)-2-[3-hydroxy-5-(sulphooxy)phenyl]ethenyl]phenyl}oxidanesulphonate | HMDB | | {4-[(e)-2-[3-hydroxy-5-(sulphooxy)phenyl]ethenyl]phenyl}oxidanesulphonic acid | HMDB |
|
|---|
| Chemical Formula | C14H12O9S2 |
|---|
| Average Molecular Mass | 388.370 g/mol |
|---|
| Monoisotopic Mass | 387.992 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | {3-hydroxy-5-[(E)-2-[4-(sulfooxy)phenyl]ethenyl]phenyl}oxidanesulfonic acid |
|---|
| Traditional Name | {3-hydroxy-5-[(E)-2-[4-(sulfooxy)phenyl]ethenyl]phenyl}oxidanesulfonic acid |
|---|
| SMILES | OC1=CC(OS(O)(=O)=O)=CC(\C=C\C2=CC=C(OS(O)(=O)=O)C=C2)=C1 |
|---|
| InChI Identifier | InChI=1S/C14H12O9S2/c15-12-7-11(8-14(9-12)23-25(19,20)21)2-1-10-3-5-13(6-4-10)22-24(16,17)18/h1-9,15H,(H,16,17,18)(H,19,20,21)/b2-1+ |
|---|
| InChI Key | DHYDAGFKCXRMSL-OWOJBTEDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as stilbenes. These are organic compounds containing a 1,2-diphenylethylene moiety. Stilbenes (C6-C2-C6 ) are derived from the common phenylpropene (C6-C3) skeleton building block. The introduction of one or more hydroxyl groups to a phenyl ring lead to stilbenoids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Stilbenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Stilbenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Stilbene
- Phenylsulfate
- Arylsulfate
- Phenoxy compound
- Styrene
- Phenol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Sulfuric acid ester
- Sulfate-ester
- Sulfuric acid monoester
- Monocyclic benzene moiety
- Benzenoid
- Organic sulfuric acid or derivatives
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-0069000000-d38579d4cc611bb35a5c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-01ej-2009200000-9b4cc8c0c00c6f70c011 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0039000000-f2a4030239b4352e5041 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0195000000-fc352d72661e5355ba46 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01t9-5950000000-18fbdad5fc916e9961cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-d0317463e8c25c201f55 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0069000000-a21cd061b78b2979bd41 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0059-3091000000-6c1f7dcd7e308ed4295a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0019000000-d77fc1ea278be934fc66 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002f-0091000000-e48c4113b389d7d6b906 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03g1-0690000000-e812b58f48ea60a0c525 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-4b720e962eaf33585c05 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-1009000000-18423343ec7e0cca0d83 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9136000000-51b915a42e18a805c9c6 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041780 |
|---|
| FooDB ID | FDB029953 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 25068570 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 46855052 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Neveu V, Perez-Jimenez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, Scalbert A: Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford). 2010;2010:bap024. doi: 10.1093/database/bap024. Epub 2010 Jan 8. |
|
|---|