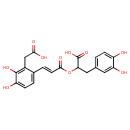| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:36:14 UTC |
|---|
| Update Date | 2016-11-09 01:22:31 UTC |
|---|
| Accession Number | CHEM041645 |
|---|
| Identification |
|---|
| Common Name | Salvianolic acid G |
|---|
| Class | Small Molecule |
|---|
| Description | Salvianolic acid G is a polyphenol metabolite detected in biological fluids (PMID: 20428313). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Salvianolate g | Generator | | 2-({3-[2-(carboxymethyl)-3,4-dihydroxyphenyl]prop-2-enoyl}oxy)-3-(3,4-dihydroxyphenyl)propanoate | HMDB |
|
|---|
| Chemical Formula | C20H18O10 |
|---|
| Average Molecular Mass | 418.351 g/mol |
|---|
| Monoisotopic Mass | 418.090 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 2-{[(2E)-3-[2-(carboxymethyl)-3,4-dihydroxyphenyl]prop-2-enoyl]oxy}-3-(3,4-dihydroxyphenyl)propanoic acid |
|---|
| Traditional Name | 2-{[(2E)-3-[2-(carboxymethyl)-3,4-dihydroxyphenyl]prop-2-enoyl]oxy}-3-(3,4-dihydroxyphenyl)propanoic acid |
|---|
| SMILES | OC(=O)CC1=C(O)C(O)=CC=C1\C=C\C(=O)OC(CC1=CC=C(O)C(O)=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C20H18O10/c21-13-4-1-10(7-15(13)23)8-16(20(28)29)30-18(26)6-3-11-2-5-14(22)19(27)12(11)9-17(24)25/h1-7,16,21-23,27H,8-9H2,(H,24,25)(H,28,29)/b6-3+ |
|---|
| InChI Key | KFCMFABBVSIHTB-ZZXKWVIFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as coumaric acids and derivatives. These are aromatic compounds containing Aromatic compounds containing a cinnamic acid moiety (or a derivative thereof) hydroxylated at the C2 (ortho-), C3 (meta-), or C4 (para-) carbon atom of the benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Cinnamic acids and derivatives |
|---|
| Sub Class | Hydroxycinnamic acids and derivatives |
|---|
| Direct Parent | Coumaric acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coumaric acid or derivatives
- Cinnamic acid ester
- 3-phenylpropanoic-acid
- 2(hydroxyphenyl)acetic acid
- Tricarboxylic acid or derivatives
- Catechol
- Styrene
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Fatty acid ester
- 1-hydroxy-4-unsubstituted benzenoid
- Benzenoid
- Fatty acyl
- Monocyclic benzene moiety
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Carboxylic acid ester
- Carboxylic acid derivative
- Carboxylic acid
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxygen compound
- Carbonyl group
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-0911000000-b10d221ddcca9a449681 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-016u-6291023000-ac576429135554fb6c3c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ue9-0923700000-a722eed7d6fb84797f3c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00gr-0913000000-2d9f1521bc2c0b8d5f8b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00g0-0900000000-eb742d1483b728a7637e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-016s-0957500000-698656ccba096f6663bc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00n4-0943000000-313fc96899e1290ad22e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-016u-1950000000-730e1237fe877938ffa9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00kb-1907500000-378c3da43827e93fef88 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00du-1923000000-d33cc20ec48d15433f12 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00fu-4904000000-64abefcec16e957752de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0629200000-65ec41c5c016de2a36d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ba-0926000000-a6756ba5af1cfa4b6a49 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00g0-0900000000-a74fa2527a8138ae1293 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041774 |
|---|
| FooDB ID | FDB029946 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00037777 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 9857888 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 11683160 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Neveu V, Perez-Jimenez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, Scalbert A: Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford). 2010;2010:bap024. doi: 10.1093/database/bap024. Epub 2010 Jan 8. | | 2. Jiang SJ, Zhu B, Zhou DD, Wang GL, Wang YX, Lin RC. Simultaneous determination of five major active depsides in the freeze-dried Dan-Shen injection by LC. Journal of Medicinal Plants Research 2011;5(10):1850-1858. [Structure] |
|
|---|