| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:35:33 UTC |
|---|
| Update Date | 2016-11-09 01:22:30 UTC |
|---|
| Accession Number | CHEM041619 |
|---|
| Identification |
|---|
| Common Name | Isoferulic acid 3-glucuronide |
|---|
| Class | Small Molecule |
|---|
| Description | Isoferulic acid 3-O-glucuronide is a polyphenol metabolite detected in biological fluids (PMID: 20428313). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
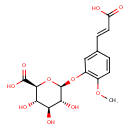
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Isoferulate 3-O-glucuronide | Generator | | (2S,3S,4S,5R,6S)-6-{5-[(1E)-2-carboxyeth-1-en-1-yl]-2-methoxyphenoxy}-3,4,5-trihydroxyoxane-2-carboxylate | HMDB | | Isoferulate 3-glucuronide | HMDB |
|
|---|
| Chemical Formula | C16H18O10 |
|---|
| Average Molecular Mass | 370.308 g/mol |
|---|
| Monoisotopic Mass | 370.090 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2S,3S,4S,5R,6S)-6-{5-[(1E)-2-carboxyeth-1-en-1-yl]-2-methoxyphenoxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| Traditional Name | (2S,3S,4S,5R,6S)-6-{5-[(1E)-2-carboxyeth-1-en-1-yl]-2-methoxyphenoxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| SMILES | COC1=C(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)C=C(\C=C\C(O)=O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C16H18O10/c1-24-8-4-2-7(3-5-10(17)18)6-9(8)25-16-13(21)11(19)12(20)14(26-16)15(22)23/h2-6,11-14,16,19-21H,1H3,(H,17,18)(H,22,23)/b5-3+/t11-,12-,13+,14-,16+/m0/s1 |
|---|
| InChI Key | SHJZLGVIOYFHCB-MBAOVNHDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenolic glycosides. These are organic compounds containing a phenolic structure attached to a glycosyl moiety. Some examples of phenolic structures include lignans, and flavonoids. Among the sugar units found in natural glycosides are D-glucose, L-Fructose, and L rhamnose. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Phenolic glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenolic glycoside
- 1-o-glucuronide
- O-glucuronide
- Glucuronic acid or derivatives
- Cinnamic acid
- Cinnamic acid or derivatives
- Coumaric acid or derivatives
- Hexose monosaccharide
- O-glycosyl compound
- Phenoxy compound
- Anisole
- Methoxybenzene
- Styrene
- Phenol ether
- Alkyl aryl ether
- Beta-hydroxy acid
- Monocyclic benzene moiety
- Benzenoid
- Dicarboxylic acid or derivatives
- Pyran
- Hydroxy acid
- Monosaccharide
- Oxane
- Secondary alcohol
- Organoheterocyclic compound
- Carboxylic acid
- Carboxylic acid derivative
- Ether
- Polyol
- Acetal
- Oxacycle
- Alcohol
- Carbonyl group
- Organic oxide
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0zi3-9175000000-4bffa5032a0119b3dbaa | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0006-1372059000-7b4407f8a87af85a556c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fba-0809000000-73927f8880312ad6c993 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002b-0901000000-3fc6034bebb10e6211f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004j-1900000000-404987ecfb4d10a54396 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014l-1709000000-cfdf4dd2009b2c6a0e97 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002f-1903000000-55bae741a25dfe015552 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002f-1900000000-861bd0f26ad0bda9cddf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fbi-0509000000-5b8c3013c941cbbcbb65 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004j-0923000000-3c57f1a73f30ae36d0c8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gyk-1910000000-93972f87218a0be3a8b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-05r0-0109000000-ebf6b9833f6b8f8533e8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4j-1948000000-7d77b44f0d00243d2c72 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000t-4931000000-6afac32f6dae6a879e63 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041747 |
|---|
| FooDB ID | FDB029917 |
|---|
| Phenol Explorer ID | 936 |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30777633 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 49844484 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Neveu V, Perez-Jimenez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, Scalbert A: Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford). 2010;2010:bap024. doi: 10.1093/database/bap024. Epub 2010 Jan 8. |
|
|---|