| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:35:15 UTC |
|---|
| Update Date | 2016-11-09 01:22:30 UTC |
|---|
| Accession Number | CHEM041604 |
|---|
| Identification |
|---|
| Common Name | Ferulic acid 4-sulfate |
|---|
| Class | Small Molecule |
|---|
| Description | A member of the class of cinnamic acids that is ferulic acid in which the phenolic hydrogen has been replaced by a sulfo group. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
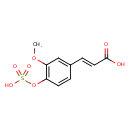
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Ferulic acid sulfate | ChEBI | | Ferulate sulfate | Generator | | Ferulate sulphate | Generator | | Ferulic acid sulfuric acid | Generator | | Ferulic acid sulphuric acid | Generator | | Ferulate 4-O-sulfate | Generator | | Ferulate 4-O-sulphate | Generator | | Ferulic acid 4-O-sulfuric acid | Generator | | Ferulic acid 4-O-sulphuric acid | Generator | | Ferulate 4-sulfate | Generator, HMDB, HMDB | | Ferulate 4-sulphate | Generator, HMDB, HMDB | | Ferulic acid 4-sulfuric acid | Generator, HMDB, HMDB | | Ferulic acid 4-sulphuric acid | Generator, HMDB, HMDB | | (2E)-3-[3-Methoxy-4-(sulfooxy)phenyl]-2-propenoic acid | HMDB | | Ferulic acid 4-O-sulfate | HMDB | | Ferulic acid-4'-sulfate | HMDB | | (E)-Ferulic acid 4-O-sulfate | HMDB | | (E)-Ferulic acid-4'-sulfate | HMDB | | trans-Ferulic acid 4-O-sulfate | HMDB | | trans-Ferulic acid-4'-sulfate | HMDB | | Ferulic acid 4-sulfate | HMDB | | (E)-Ferulic acid 4-O-sulphate | HMDB | | (E)-Ferulic acid-4'-sulphate | HMDB | | (E)-Ferulic acid-4’-sulfate | HMDB | | (E)-Ferulic acid-4’-sulphate | HMDB | | Ferulic acid 4-O-sulphate | HMDB | | Ferulic acid 4-sulphate | HMDB | | Ferulic acid-4'-sulphate | HMDB | | Ferulic acid-4’-sulfate | HMDB | | Ferulic acid-4’-sulphate | HMDB | | trans-Ferulic acid 4-O-sulphate | HMDB | | trans-Ferulic acid-4'-sulphate | HMDB | | trans-Ferulic acid-4’-sulfate | HMDB | | trans-Ferulic acid-4’-sulphate | HMDB |
|
|---|
| Chemical Formula | C10H10O7S |
|---|
| Average Molecular Mass | 274.247 g/mol |
|---|
| Monoisotopic Mass | 274.015 g/mol |
|---|
| CAS Registry Number | 86321-29-1 |
|---|
| IUPAC Name | (2E)-3-[3-methoxy-4-(sulfooxy)phenyl]prop-2-enoic acid |
|---|
| Traditional Name | (2E)-3-[3-methoxy-4-(sulfooxy)phenyl]prop-2-enoic acid |
|---|
| SMILES | COC1=C(OS(O)(=O)=O)C=CC(\C=C\C(O)=O)=C1 |
|---|
| InChI Identifier | InChI=1S/C10H10O7S/c1-16-9-6-7(3-5-10(11)12)2-4-8(9)17-18(13,14)15/h2-6H,1H3,(H,11,12)(H,13,14,15)/b5-3+ |
|---|
| InChI Key | PZPATWACAAOHTJ-HWKANZROSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as coumaric acids and derivatives. These are aromatic compounds containing Aromatic compounds containing a cinnamic acid moiety (or a derivative thereof) hydroxylated at the C2 (ortho-), C3 (meta-), or C4 (para-) carbon atom of the benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Cinnamic acids and derivatives |
|---|
| Sub Class | Hydroxycinnamic acids and derivatives |
|---|
| Direct Parent | Coumaric acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cinnamic acid
- Coumaric acid or derivatives
- Phenylsulfate
- Arylsulfate
- Phenoxy compound
- Anisole
- Methoxybenzene
- Phenol ether
- Styrene
- Alkyl aryl ether
- Monocyclic benzene moiety
- Benzenoid
- Sulfuric acid ester
- Sulfate-ester
- Sulfuric acid monoester
- Organic sulfuric acid or derivatives
- Monocarboxylic acid or derivatives
- Ether
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxygen compound
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4m-1980000000-0a04a82fe28e63433e61 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00gi-6194000000-af9906ac00d0c397e1c1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-cbbeeecc312476cf7b4c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056s-1790000000-c30f07543dbbb35fc47e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-003r-8920000000-1c33c8ea0fa1f33c6ead | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-5f31290999c27432202e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00bc-0950000000-498bb5d6540050ba1f92 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0059-4900000000-3eb0d6bf9f242cb7be06 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-31ce769cc85e2eadefca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004j-6190000000-6e72be15cb6746c0823e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9400000000-adf92ccf0dc6414f1c26 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0290000000-1af5b5745da90ec7f9fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0920000000-f89ce8310fc96f9e6937 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01q9-2900000000-277ba2ff8bec524d449e | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029200 |
|---|
| FooDB ID | FDB029900 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4878542 |
|---|
| ChEBI ID | 133508 |
|---|
| PubChem Compound ID | 6305574 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. https://www.ncbi.nlm.nih.gov/pubmed/?term=21417257 | | 2. https://www.ncbi.nlm.nih.gov/pubmed/?term=24503197 | | 3. https://www.ncbi.nlm.nih.gov/pubmed/?term=25787755 | | 4. Menozzi-Smarrito C, Wong CC, Meinl W, Glatt H, Fumeaux R, Munari C, Robert F, Williamson G, Barron D: First chemical synthesis and in vitro characterization of the potential human metabolites 5-o-feruloylquinic acid 4'-sulfate and 4'-O-glucuronide. J Agric Food Chem. 2011 May 25;59(10):5671-6. doi: 10.1021/jf200272m. Epub 2011 Apr 21. | | 5. Amin HP, Czank C, Raheem S, Zhang Q, Botting NP, Cassidy A, Kay CD: Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol Nutr Food Res. 2015 Jun;59(6):1095-106. doi: 10.1002/mnfr.201400803. Epub 2015 Apr 30. | | 6. Ounnas F, Prive F, Salen P, Gaci N, Tottey W, Calani L, Bresciani L, Lopez-Gutierrez N, Hazane-Puch F, Laporte F, Brugere JF, Del Rio D, Demeilliers C, de Lorgeril M: Whole Rye Consumption Improves Blood and Liver n-3 Fatty Acid Profile and Gut Microbiota Composition in Rats. PLoS One. 2016 Feb 10;11(2):e0148118. doi: 10.1371/journal.pone.0148118. eCollection 2016. | | 7. Publications of the University of Eastern Finland. Dissertations in Health Sciences., no 510 |
|
|---|